N-1 Perfusion Platform Development Using a Capacitance Probe for Biomanufacturing
- PMID: 35447688
- PMCID: PMC9029935
- DOI: 10.3390/bioengineering9040128
N-1 Perfusion Platform Development Using a Capacitance Probe for Biomanufacturing
Abstract
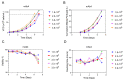
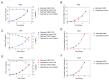
Fed-batch process intensification with a significantly shorter culture duration or higher titer for monoclonal antibody (mAb) production by Chinese hamster ovary (CHO) cells can be achieved by implementing perfusion operation at the N-1 stage for biomanufacturing. N-1 perfusion seed with much higher final viable cell density (VCD) than a conventional N-1 batch seed can be used to significantly increase the inoculation VCD for the subsequent fed-batch production (referred as N stage), which results in a shorter cell growth phase, higher peak VCD, or higher titer. In this report, we incorporated a process analytical technology (PAT) tool into our N-1 perfusion platform, using an in-line capacitance probe to automatically adjust the perfusion rate based on real-time VCD measurements. The capacitance measurements correlated linearly with the offline VCD at all cell densities tested (i.e., up to 130 × 106 cells/mL). Online control of the perfusion rate via the cell-specific perfusion rate (CSPR) decreased media usage by approximately 25% when compared with a platform volume-specific perfusion rate approach and did not lead to any detrimental effects on cell growth. This PAT tool was applied to six mAbs, and a platform CSPR of 0.04 nL/cell/day was selected, which enabled rapid growth and maintenance of high viabilities for four of six cell lines. In addition, small-scale capacitance data were used in the scaling-up of N-1 perfusion processes in the pilot plant and in the GMP manufacturing suite. Implementing a platform approach based on capacitance measurements to control perfusion rates led to efficient process development of perfusion N-1 for supporting high-density CHO cell cultures for the fed-batch process intensification.
Keywords: Chinese hamster ovary (CHO); capacitance; cell-specific perfusion rate (CSPR); in-line probe; monoclonal antibody (mAb); perfusion N-1; platform; process analytical technologies (PAT); process intensification; scale-up.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Müller D., Klein L., Lemke J., Schulze M., Kruse T., Saballus M., Matuszczyk J., Kampmann M., Zijlstra G. Process intensification in the biopharma industry: Improving efficiency of protein manufacturing processes from development to production scale using synergistic approaches. Chem. Eng. Process.—Process Intensif. 2022;171:108727. doi: 10.1016/j.cep.2021.108727. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

