Lactide: Production Routes, Properties, and Applications
- PMID: 35447724
- PMCID: PMC9032396
- DOI: 10.3390/bioengineering9040164
Lactide: Production Routes, Properties, and Applications
Abstract
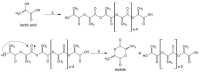
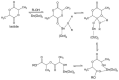
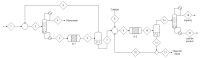
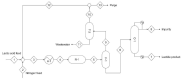
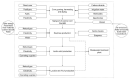
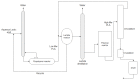
Lactide dimer is an important monomer produced from lactic acid dehydration, followed by the prepolymer depolymerization process, and subsequent purification. As lactic acid is a chiral molecule, lactide can exist in three isomeric forms: L-, D-, and meso-lactide. Due to its time-consuming synthesis and the need for strict temperature and pressure control, catalyst use, low selectivity, high energy cost, and racemization, the value of a high purity lactide has a high cost in the market; moreover, little is found in scientific articles about the monomer synthesis. Lactide use is mainly for the synthesis of high molar mass poly(lactic acid) (PLA), applied as bio-based material for medical applications (e.g., prostheses and membranes), drug delivery, and hydrogels, or combined with other polymers for applications in packaging. This review elucidates the configurations and conditions of syntheses mapped for lactide production, the main properties of each of the isomeric forms, its industrial production, as well as the main applications in the market.
Keywords: applications; industrial processes; l-lactide; market costs; synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

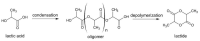

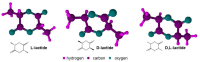
References
-
- Ghadamyari M., Chaemchuen S., Zhou K., Dusselier M., Sels B.F., Mousavi B., Verpoort F. One-Step Synthesis of Stereo-Pure L,L Lactide from L-Lactic Acid. Catal. Commun. 2018;114:33–36. doi: 10.1016/j.catcom.2018.06.003. - DOI
-
- Ehsani M., Khodabakhshi K., Asgari M. Lactide Synthesis Optimization: Investigation of the Temperature, Catalyst and Pressure Effects. E-Polymers. 2014;14:353–361. doi: 10.1515/epoly-2014-0055. - DOI
-
- Shen L., Haufe J., Patel M.K. Product Overview and Market Projection of Emerging Bio-Based Plastics. Universiteit Utrecht; Utrecht, The Netherlands: 2009.
Publication types
Grants and funding
- grant #2015/20630-4 São Paulo Research Foundation and grant #2021/05719-0 São Paulo Research Foundation/National Council for Scientific and Technological Development (Conselho Nacional de Desenvol-vimento Científico e Tecnológico - CNPq, Brazil), the Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de
- UIDB/04469/2020 (strategic fund)/the Portuguese Science and Technology Foundation (FCT/MCT) and European Funds (PRODER/COMPETE)
- (C.2020) granted to PS/Fundação Carolina (Movilidad de profesorado Brasil-España, Movilidad. Estancias de Investigación
LinkOut - more resources
Full Text Sources
Research Materials

