Assessing Near-Infrared Spectroscopy (NIRS) for Evaluation of Aedes aegypti Population Age Structure
- PMID: 35447802
- PMCID: PMC9029691
- DOI: 10.3390/insects13040360
Assessing Near-Infrared Spectroscopy (NIRS) for Evaluation of Aedes aegypti Population Age Structure
Abstract
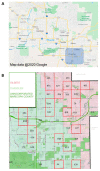
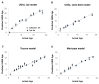
Given that older Aedes aegypti (L.) mosquitoes typically pose the greatest risk of pathogen transmission, the capacity to age grade wild Ae. aegypti mosquito populations would be a valuable tool in monitoring the potential risk of arboviral transmission. Here, we compared the effectiveness of near-infrared spectroscopy (NIRS) to age grade field-collected Ae. aegypti with two alternative techniques-parity analysis and transcript abundance of the age-associated gene SCP1. Using lab-reared mosquitoes of known ages from three distinct populations maintained as adults under laboratory or semi-field conditions, we developed and validated four NIRS models for predicting the age of field-collected Ae. aegypti. To assess the accuracy of these models, female Ae. aegypti mosquitoes were collected from Maricopa County, AZ, during the 2017 and 2018 monsoon season, and a subset were age graded using the three different age-grading techniques. For both years, each of the four NIRS models consistently graded parous mosquitoes as significantly older than nulliparous mosquitoes. Furthermore, a significant positive linear association occurred between SCP1 and NIRS age predictions in seven of the eight year/model combinations, although considerable variation in the predicted age of individual mosquitoes was observed. Our results suggest that although the NIRS models were not adequate in determining the age of individual field-collected mosquitoes, they have the potential to quickly and cost effectively track changes in the age structure of Ae. aegypti populations across locations and over time.
Keywords: NIRS; SCP1; Sonoran; aging; mosquito; parity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Dentinova T. Age-Grouping Methods in Diptera of Medical Importance. World Health Organization; Geneva, Switzerland: 1962. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

