The Emerging Evidence for a Protective Role of Fucoidan from Laminaria japonica in Chronic Kidney Disease-Triggered Cognitive Dysfunction
- PMID: 35447931
- PMCID: PMC9025131
- DOI: 10.3390/md20040258
The Emerging Evidence for a Protective Role of Fucoidan from Laminaria japonica in Chronic Kidney Disease-Triggered Cognitive Dysfunction
Abstract
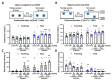
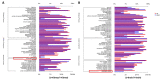
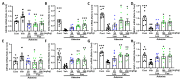
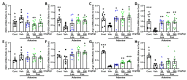
This study aimed to explore the mechanism of fucoidan in chronic kidney disease (CKD)-triggered cognitive dysfunction. The adenine-induced ICR strain CKD mice model was applied, and RNA-Seq was performed for differential gene analysis between aged-CKD and normal mice. As a result, fucoidan (100 and 200 mg kg-1) significantly reversed adenine-induced high expression of urea, uric acid in urine, and creatinine in serum, as well as the novel object recognition memory and spatial memory deficits. RNA sequencing analysis indicated that oxidative and inflammatory signaling were involved in adenine-induced kidney injury and cognitive dysfunction; furthermore, fucoidan inhibited oxidative stress via GSK3β-Nrf2-HO-1 signaling and ameliorated inflammatory response through regulation of microglia/macrophage polarization in the kidney and hippocampus of CKD mice. Additionally, we clarified six hallmarks in the hippocampus and four in the kidney, which were correlated with CKD-triggered cognitive dysfunction. This study provides a theoretical basis for the application of fucoidan in the treatment of CKD-triggered memory deficits.
Keywords: GSK3β-Nrf2-HO-1 signaling; chronic kidney disease; cognitive dysfunction; fucoidan; microglial polarization; neuroinflammation; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Hooper S.R., Gerson A.C., Butler R.W., Gipson D.S., Mendley S.R., Lande M.B., Shinnar S., Wentz A., Matheson M., Cox C. Neurocognitive functioning of children and adolescents with mild-to-moderate chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011;6:1824–1830. doi: 10.2215/CJN.09751110. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 81803753/National Natural Science Foundation of China
- grant number 2018A01046/Special Funds for Science and Technology Development of Zhanjiang City
- 2020A1515010779/Natural Science Foundation of Guangdong Province of China
- R19035/Scientific Research Foundation of Guangdong Ocean University
- 2021KCXTD021/The Innovative Team Program of High Education of Guangdong Province
LinkOut - more resources
Full Text Sources
Medical
Research Materials

