Tumor Microenvironment in Glioma Invasion
- PMID: 35448036
- PMCID: PMC9031400
- DOI: 10.3390/brainsci12040505
Tumor Microenvironment in Glioma Invasion
Abstract
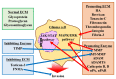
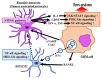
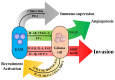
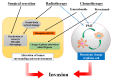
A major malignant trait of gliomas is their remarkable infiltration capacity. When glioma develops, the tumor cells have already reached the distant part. Therefore, complete removal of the glioma is impossible. Recently, research on the involvement of the tumor microenvironment in glioma invasion has advanced. Local hypoxia triggers cell migration as an environmental factor. The transcription factor hypoxia-inducible factor (HIF) -1α, produced in tumor cells under hypoxia, promotes the transcription of various invasion related molecules. The extracellular matrix surrounding tumors is degraded by proteases secreted by tumor cells and simultaneously replaced by an extracellular matrix that promotes infiltration. Astrocytes and microglia become tumor-associated astrocytes and glioma-associated macrophages/microglia, respectively, in relation to tumor cells. These cells also promote glioma invasion. Interactions between glioma cells actively promote infiltration of each other. Surgery, chemotherapy, and radiation therapy transform the microenvironment, allowing glioma cells to invade. These findings indicate that the tumor microenvironment may be a target for glioma invasion. On the other hand, because the living body actively promotes tumor infiltration in response to the tumor, it is necessary to reconsider whether the invasion itself is friend or foe to the brain.
Keywords: glioma; invasion; microenvironment.
Conflict of interest statement
All authors declare no conflict of interest for this article.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

