Antiarrhythmic Effects of Vernakalant in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes from a Patient with Short QT Syndrome Type 1
- PMID: 35448088
- PMCID: PMC9032933
- DOI: 10.3390/jcdd9040112
Antiarrhythmic Effects of Vernakalant in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes from a Patient with Short QT Syndrome Type 1
Abstract
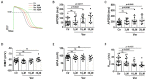
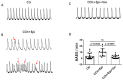
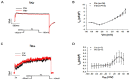
(1) Background: Short QT syndrome (SQTS) may result in sudden cardiac death. So far, no drugs, except quinidine, have been demonstrated to be effective in some patients with SQTS type 1 (SQTS1). This study was designed to examine the potential effectiveness of vernakalant for treating SQTS1 patients, using human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) from a patient with SQTS1. (2) Methods: Patch clamp and calcium imaging techniques were used to examine the drug effects. (3) Results: Vernakalant prolonged the action potential duration (APD) in hiPSC-CMs from a SQTS1-patient (SQTS1-hiPSC-CMs). In spontaneously beating SQTS1-hiPSC-CMs, vernakalant reduced the arrhythmia-like events induced by carbachol plus epinephrine. Vernakalant failed to suppress the hERG channel currents but reduced the outward small-conductance calcium-activated potassium channel current. In addition, it enhanced Na/Ca exchanger currents and late sodium currents, in agreement with its APD-prolonging effect. (4) Conclusions: The results demonstrated that vernakalant can prolong APD and reduce arrhythmia-like events in SQTS1-hiPSC-CMs and may be a candidate drug for treating arrhythmias in SQTS1-patients.
Keywords: antiarrhythmic drugs; arrhythmias; human-induced pluripotent stem cell-derived cardiomyocytes; short QT syndrome; vernakalant.
Conflict of interest statement
All authors declared no competing interest for this work.
Figures




References
-
- Thorsen K., Dam V.S., Kjaer-Sorensen K., Pedersen L.N., Skeberdis V.A., Jurevičius J., Treinys R., Petersen I.M.B.S., Nielsen M.S., Oxvig C., et al. Loss-of-activity-mutation in the cardiac chloride-bicarbonate exchanger AE3 causes short QT syndrome. Nat. Commun. 2017;8:1696. doi: 10.1038/s41467-017-01630-0. - DOI - PMC - PubMed
-
- Priori S.G., Blomstrom-Lundqvist C., Mazzanti A., Blom N., Borggrefe M., Camm J., Elliott P.M., Fitzsimons D., Hatala R., Hindricks G., et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC) Eur. Heart J. 2015;36:2793–2867. - PubMed
LinkOut - more resources
Full Text Sources

