Self-Assembling Peptide Hydrogels as Functional Tools to Tackle Intervertebral Disc Degeneration
- PMID: 35448112
- PMCID: PMC9028266
- DOI: 10.3390/gels8040211
Self-Assembling Peptide Hydrogels as Functional Tools to Tackle Intervertebral Disc Degeneration
Abstract
Low back pain (LBP), caused by intervertebral disc (IVD) degeneration, is a major contributor to global disability. In its healthy state, the IVD is a tough and well-hydrated tissue, able to act as a shock absorber along the spine. During degeneration, the IVD is hit by a cell-driven cascade of events, which progressively lead to extracellular matrix (ECM) degradation, chronic inflammation, and pain. Current treatments are divided into palliative care (early stage degeneration) and surgical interventions (late-stage degeneration), which are invasive and poorly efficient in the long term. To overcome these limitations, alternative tissue engineering and regenerative medicine strategies, in which soft biomaterials are used as injectable carriers of cells and/or biomolecules to be delivered to the injury site and restore tissue function, are currently being explored. Self-assembling peptide hydrogels (SAPHs) represent a promising class of de novo synthetic biomaterials able to merge the strengths of both natural and synthetic hydrogels for biomedical applications. Inherent features, such as shear-thinning behaviour, high biocompatibility, ECM biomimicry, and tuneable physiochemical properties make these hydrogels appropriate and functional tools to tackle IVD degeneration. This review will describe the pathogenesis of IVD degeneration, list biomaterials requirements to attempt IVD repair, and focus on current peptide hydrogel materials exploited for this purpose.
Keywords: intervertebral disc; self-assembling peptide hydrogels; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


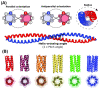
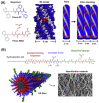

References
-
- Neidlinger-Wilke C., Galbusera F., Pratsinis H., Mavrogonatou E., Mietsch A., Kletsas D., Wilke H.-J. Mechanical loading of the intervertebral disc: From the macroscopic to the cellular level. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2014;23((Suppl. S3)):S333–S343. doi: 10.1007/s00586-013-2855-9. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

