Poly(ethylene oxide) Is Positively Charged in Aqueous Solutions
- PMID: 35448114
- PMCID: PMC9029200
- DOI: 10.3390/gels8040213
Poly(ethylene oxide) Is Positively Charged in Aqueous Solutions
Abstract
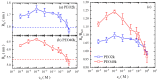
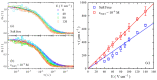
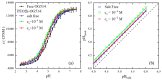
There have been controversies about the binding of cations to poly(ethylene oxide) (PEO) chains in aqueous solutions. In the current study, single molecular evidence of charging PEO chains by cation binding in aqueous solutions is provided. From the adoption of the photon-counting histogram method, it is discovered that the local pH value at the vicinity of the PEO chain is higher than the bulk solution, showing that the PEO chain is positively charged. Such a situation exists with and without the presence of salt (NaCl) in the solution, presumably due to the binding of cations, such as hydronium and sodium ions. Single molecular electrophoresis experiments using fluorescence correlation spectroscopy demonstrate that the PEO chains are weakly charged with a charging extent of ~5%. In comparison to the salt-free condition, the addition of external salt (NaCl) at moderate concentrations further charges the chain. The charging causes the PEO chains to expand and a further increase in the salt concentration causes the chain to shrink, exhibiting a polyelectrolyte-like behavior, demonstrated by the hydrodynamic radii of a single PEO chain. The effect of ion identity is discovered with alkali cations, with the order of the charging capacity of Li+ < Na+ < Cs+ < K+.
Keywords: cations; charged; polyethylene oxide; single molecule fluorescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Laser light scattering and isothermal titration calorimetric studies of poly(ethylene oxide) aqueous solution in presence of sodium dodecyl sulfate.J Colloid Interface Sci. 2005 Dec 1;292(1):79-85. doi: 10.1016/j.jcis.2005.05.079. Epub 2005 Jul 18. J Colloid Interface Sci. 2005. PMID: 16026792
-
Poly(ethylene oxide) helical conformation and alkali metal cation selectivity studied using electrospray ionization mass spectrometry.Rapid Commun Mass Spectrom. 2020 May 15;34(9):e8719. doi: 10.1002/rcm.8719. Rapid Commun Mass Spectrom. 2020. PMID: 31899562
-
A small-angle neutron scattering study of poly(ethylene oxide) microstructure in aqueous poly(styrenesulfonate sodium) solutions.J Colloid Interface Sci. 2011 Jun 1;358(1):226-9. doi: 10.1016/j.jcis.2011.03.015. Epub 2011 Mar 10. J Colloid Interface Sci. 2011. PMID: 21440903
-
Selective adsorption of poly(ethylene oxide) onto a charged surface mediated by alkali metal ions.Langmuir. 2008 Feb 19;24(4):1570-6. doi: 10.1021/la702514j. Epub 2007 Dec 7. Langmuir. 2008. PMID: 18062712
-
pH responsiveness of dendrimer-like poly(ethylene oxide)s.J Am Chem Soc. 2006 Sep 6;128(35):11551-62. doi: 10.1021/ja0631605. J Am Chem Soc. 2006. PMID: 16939279
Cited by
-
Editorial on Special Issue: "Dynamics of Gels and Its Applications".Gels. 2022 Dec 8;8(12):805. doi: 10.3390/gels8120805. Gels. 2022. PMID: 36547329 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

