Novel Formulation of Fusidic Acid Incorporated into a Myrrh-Oil-Based Nanoemulgel for the Enhancement of Skin Bacterial Infection Treatment
- PMID: 35448146
- PMCID: PMC9027726
- DOI: 10.3390/gels8040245
Novel Formulation of Fusidic Acid Incorporated into a Myrrh-Oil-Based Nanoemulgel for the Enhancement of Skin Bacterial Infection Treatment
Abstract
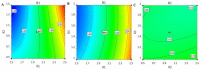
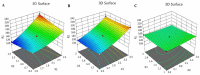
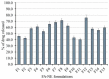
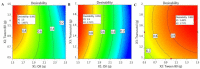
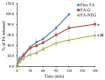
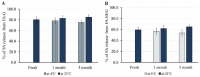
Fusidic acid (FA) is renowned as an effective bacteriostatic agent obtained from the fungus Fusidium coccineum, used for treating various eye and skin disorders. The objective of the present study was to develop, characterize, and evaluate the antibacterial activity of a novel FA nanoemulgel for topical skin application. Primarily, various fusidic acid nanoemulsion formulations were fabricated using different concentrations of myrrh essential oil, Tween 80 as a surfactant, and Transcutol® P as a co-surfactant. A Box−Behnken design was employed to select the optimized FA nanoemulsion formulation, based on the evaluated particle size and % of in vitro release as dependent variables. The optimized formula was incorporated within a hydrogel to obtain an FA nanoemulgel (FA-NEG) preparation. The formulated FA-NEG was evaluated for its visual appearance, pH, viscosity, and spreadability, compared to its corresponding prepared fusidic acid gel. In vitro release, kinetic study, and ex vivo drug permeation were implemented, followed by formulation stability testing. The FA-NEG exhibited a smooth and homogeneous appearance, pH value (6.61), viscosity (25,265 cP), and spreadability (33.6 mm), which were all good characteristics for appropriate topical application. A total of 59.3% of FA was released from the FA-NEG after 3 h. The ex vivo skin permeability of the FA-NEG was significantly enhanced by 3.10 ± 0.13-fold, showing SSTF of 111.2 ± 4.5 µg/cm2·h when compared to other formulations under investigation (p < 0.05). No irritation was observed upon applying the FA-NEG to animal skin. Eventually, it was revealed that the FA-NEG displayed improved antibacterial activity against a wide variety of bacteria when compared to its corresponding FA gel and marketed cream, indicating the prospective antibacterial effect of myrrh essential oil. In conclusion, the recommended formulation offers a promising antibacterial approach for skin infections.
Keywords: antibacterial; fusidic acid; myrrh essential oil; nanoemulgel; optimization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
An Overview of Nanoemulgels for Bioavailability Enhancement in Inflammatory Conditions via Topical Delivery.Pharmaceutics. 2023 Apr 7;15(4):1187. doi: 10.3390/pharmaceutics15041187. Pharmaceutics. 2023. PMID: 37111672 Free PMC article. Review.
-
Antibacterial Activity of Fusidic Acid and Sodium Fusidate Nanoparticles Incorporated in Pine Oil Nanoemulgel.Int J Nanomedicine. 2019 Dec 2;14:9411-9421. doi: 10.2147/IJN.S229557. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31819440 Free PMC article.
-
Enhancement of Curcumin Anti-Inflammatory Effect via Formulation into Myrrh Oil-Based Nanoemulgel.Polymers (Basel). 2021 Feb 14;13(4):577. doi: 10.3390/polym13040577. Polymers (Basel). 2021. PMID: 33672981 Free PMC article.
-
Anti-ageing Potential of Metformin Loaded Fennel Oil-Based Nanoemulgel: A Promising Novel Approach for Ultraviolet B-Induced Ageing.Int J Pharm. 2025 May 15;676:125559. doi: 10.1016/j.ijpharm.2025.125559. Epub 2025 Apr 4. Int J Pharm. 2025. PMID: 40189168
-
Potential and future scope of nanoemulgel formulation for topical delivery of lipophilic drugs.Int J Pharm. 2017 Jun 30;526(1-2):353-365. doi: 10.1016/j.ijpharm.2017.04.068. Epub 2017 Apr 28. Int J Pharm. 2017. PMID: 28461261 Review.
Cited by
-
Date Palm Extract (Phoenix dactylifera) Encapsulated into Palm Oil Nanolipid Carrier for Prospective Antibacterial Influence.Plants (Basel). 2023 Oct 25;12(21):3670. doi: 10.3390/plants12213670. Plants (Basel). 2023. PMID: 37960029 Free PMC article.
-
Development and Optimization of Nigella sativa Nanoemulsion Loaded with Pioglitazone for Hypoglycemic Effect.Polymers (Basel). 2022 Jul 26;14(15):3021. doi: 10.3390/polym14153021. Polymers (Basel). 2022. PMID: 35893989 Free PMC article.
-
Development of Soft Luliconazole Invasomes Gel for Effective Transdermal Delivery: Optimization to In-Vivo Antifungal Activity.Gels. 2023 Aug 3;9(8):626. doi: 10.3390/gels9080626. Gels. 2023. PMID: 37623081 Free PMC article.
-
Development and evaluation of nanoemulsion gel loaded with bioactive extract of Cucumis melo var. agrestis: A novel approach for enhanced skin permeability and antifungal activity.Heliyon. 2024 Jul 24;10(15):e35069. doi: 10.1016/j.heliyon.2024.e35069. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39170221 Free PMC article.
-
An Overview of Nanoemulgels for Bioavailability Enhancement in Inflammatory Conditions via Topical Delivery.Pharmaceutics. 2023 Apr 7;15(4):1187. doi: 10.3390/pharmaceutics15041187. Pharmaceutics. 2023. PMID: 37111672 Free PMC article. Review.
References
-
- Aksu N.B., Yozgatlı V., Okur M.E., Ayla Ş., Yoltaş A., Üstündağ Okur N. Preparation and evaluation of QbD based fusidic acid loaded in situ gel formulations for burn wound treatment. J. Drug Deliv. Sci. Technol. 2019;52:110–121. doi: 10.1016/j.jddst.2019.04.015. - DOI
-
- Patra J.K., Das G., Fraceto L.F., Campos E.V.R., Rodriguez-Torres M.D.P., Acosta-Torres L.S., Diaz-Torres L.A., Grillo R., Swamy M.K., Sharma S., et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018;16:71. doi: 10.1186/s12951-018-0392-8. - DOI - PMC - PubMed
-
- Elsewedy H.S., Al-Dhubiab B.E., Mahdy M.A., Elnahas H.M. Basic Concepts of Nanoemulsion and its Potential application in Pharmaceutical, Cosmeceutical and Nutraceutical fields. Res. J. Pharm. Technol. 2021;14:3938–3946. doi: 10.52711/0974-360X.2021.00684. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

