The Impact of COVID-19 on Academic Cancer Clinical Trials in Canada and the Initial Response from Cancer Centers
- PMID: 35448171
- PMCID: PMC9026465
- DOI: 10.3390/curroncol29040197
The Impact of COVID-19 on Academic Cancer Clinical Trials in Canada and the Initial Response from Cancer Centers
Abstract
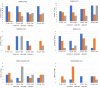
The COVID-19 pandemic resulted in temporary holds placed on new trial startups, patient recruitment and follow up visits for trials which contributed to major disruptions in cancer center trial unit operations. To assess the impact, the Canadian Cancer Clinical Trials Network (3CTN) members participated in regional meetings and a survey to understand the impact of the pandemic to academic cancer clinical trials (ACCT) activity, cancer trial unit operations and supports needed for post-pandemic recovery. Trial performance and recruitment data collected from 1 April 2020-31 March 2021 was compared to the same period in previous years. From 1 April-30 June 2020, patient recruitment decreased by 67.5% and trial site activations decreased by 81% compared to the same period in 2019. Recovery to reopening and recruitment of ACCTs began after three months, which was faster than initially projected. However, ongoing COVID-19 impacts on trial unit staffing and operations continue to contribute to delayed trial activations, lower patient recruitment and may further strain centers' capacity for participation in academic-sponsored trials.
Keywords: COVID-19 pandemic impact; Canadian cancer clinical trials; academic cancer trials; trial site activations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Annual Report 2019–2020. Canadian Cancer Clinical Trials Network; Toronto, ON, Canada: 2020.
-
- Waterhouse D.M., Harvey R.D., Hurley P., Levit L.A., Kim E.S., Klepin H.D., Mileham K.F., Nowakowski G., Schenkel C., Davis C., et al. Early Impact of COVID-19 on the Conduct of Oncology Clinical Trials and Long-Term Opportunities for Transformation: Findings From an American Society of Clinical Oncology Survey. JCO Oncol. Pract. 2020;16:417–421. doi: 10.1200/OP.20.00275. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

