Cutaneous Adverse Events Associated with Immune Checkpoint Inhibitors: A Review Article
- PMID: 35448208
- PMCID: PMC9032875
- DOI: 10.3390/curroncol29040234
Cutaneous Adverse Events Associated with Immune Checkpoint Inhibitors: A Review Article
Abstract
Immune checkpoint inhibitors (ICIs) have emerged as novel options that are effective in treating various cancers. They are monoclonal antibodies that target cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed cell death 1 (PD-1), and programmed cell death-ligand 1 (PD-L1). However, activation of the immune systems through ICIs may concomitantly trigger a constellation of immunologic symptoms and signs, termed immune-related adverse events (irAEs), with the skin being the most commonly involved organ. The dermatologic toxicities are observed in nearly half of the patients treated with ICIs, mainly in the form of maculopapular rash and pruritus. In the majority of cases, these cutaneous irAEs are self-limiting and manageable, and continuation of the ICIs is possible. This review provides an overview of variable ICI-mediated dermatologic reactions and describes the clinical and histopathologic presentation. Early and accurate diagnosis, recognition of severe toxicities, and appropriate management are key goals to achieve the most favorable outcomes and quality of life in cancer patients.
Keywords: anti-CTLA-4 inhibitor; anti-PD-1 inhibitor; anti-PD-L1 inhibitor; cutaneous immune-related adverse event; immune checkpoint inhibitor; immune-related adverse event.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

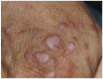
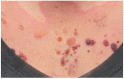
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

