Visualising the Emerging Platform of Using Microalgae as a Sustainable Bio-Factory for Healthy Lipid Production through Biocompatible AIE Probes
- PMID: 35448268
- PMCID: PMC9029145
- DOI: 10.3390/bios12040208
Visualising the Emerging Platform of Using Microalgae as a Sustainable Bio-Factory for Healthy Lipid Production through Biocompatible AIE Probes
Abstract
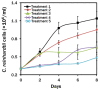
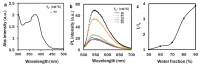
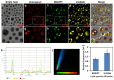
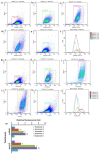
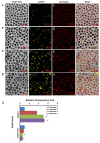
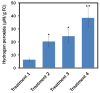
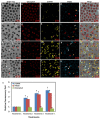
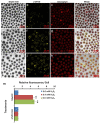
Nowadays, a particular focus is using microalgae to get high-valued health beneficiary lipids. The precise localisation of the lipid droplets (LDs) and biochemical changes are crucial to portray the lipid production strategy in algae, but it requires an in vivo tool to rapidly visualise LD distribution. As a novel strategy, this study focuses on detecting lipid bioaccumulation in a green microalga, Chlamydomonas reinhardtii using the aggregation-induced emission (AIE) based probe, 2-DPAN (C24H18N2O). As the messenger molecule and stress biomarker, hydrogen peroxide (H2O2) activity was detected in lipid synthesis with the AIE probe, TPE-BO (C38H42B2O4). Distinctive LDs labelled with 2-DPAN have elucidated the lipid inducing conditions, where more health beneficiary α-linolenic acid has been produced. TPE-BO labelled H2O2 have clarified the involvement of H2O2 during lipid biogenesis. The co-staining procedure with traditional green BODIPY dye and red chlorophyll indicates that 2-DPAN is suitable for multicolour LD imaging. Compared with BODIPY, 2-DPAN was an efficient sample preparation technique without the washing procedure. Thus, 2-DPAN could improve traditional fluorescent probes currently used for lipid imaging. In addition, the rapid, wash-free, multicolour AIE-based in vivo probe in the study of LDs with 2-DPAN could advance the research of lipid production in microalgae.
Keywords: aggregation-induced emission; green microalgae; health beneficiaries; healthy lipid; visualisation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Aggregation-Induced Emission Photosensitizer Boosting Algal Growth and Lipid Accumulation.Small. 2024 Nov;20(47):e2402463. doi: 10.1002/smll.202402463. Epub 2024 Aug 19. Small. 2024. PMID: 39161188 Free PMC article.
-
Enhancement of Growth and Lipid Production in Microalgae Using Aggregation-Induced Emission Based Luminescent Material for Sustainable Food and Fuel.Chem Asian J. 2025 Jan 2;20(1):e202401077. doi: 10.1002/asia.202401077. Epub 2024 Nov 9. Chem Asian J. 2025. PMID: 39375156
-
A highly selective AIE fluorogen for lipid droplet imaging in live cells and green algae.J Mater Chem B. 2014 Apr 14;2(14):2013-2019. doi: 10.1039/c3tb21675f. Epub 2014 Mar 4. J Mater Chem B. 2014. PMID: 32261636
-
Microalgal lipid droplets: composition, diversity, biogenesis and functions.Plant Cell Rep. 2015 Apr;34(4):545-55. doi: 10.1007/s00299-014-1711-7. Epub 2014 Nov 30. Plant Cell Rep. 2015. PMID: 25433857 Review.
-
Metabolism of acyl-lipids in Chlamydomonas reinhardtii.Plant J. 2015 May;82(3):504-522. doi: 10.1111/tpj.12787. Epub 2015 Mar 3. Plant J. 2015. PMID: 25660108 Review.
Cited by
-
Aggregation-Induced Emission (AIE), Life and Health.ACS Nano. 2023 Aug 8;17(15):14347-14405. doi: 10.1021/acsnano.3c03925. Epub 2023 Jul 24. ACS Nano. 2023. PMID: 37486125 Free PMC article. Review.
-
The need for smart microalgal bioprospecting.Nat Prod Bioprospect. 2025 Jan 16;15(1):7. doi: 10.1007/s13659-024-00487-3. Nat Prod Bioprospect. 2025. PMID: 39815030 Free PMC article. Review.
-
Aggregation-Induced Emission Photosensitizer Boosting Algal Growth and Lipid Accumulation.Small. 2024 Nov;20(47):e2402463. doi: 10.1002/smll.202402463. Epub 2024 Aug 19. Small. 2024. PMID: 39161188 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

