Antibiotic Isoflavonoids, Anthraquinones, and Pterocarpanoids from Pigeon Pea (Cajanus cajan L.) Seeds against Multidrug-Resistant Staphylococcus aureus
- PMID: 35448466
- PMCID: PMC9030341
- DOI: 10.3390/metabo12040279
Antibiotic Isoflavonoids, Anthraquinones, and Pterocarpanoids from Pigeon Pea (Cajanus cajan L.) Seeds against Multidrug-Resistant Staphylococcus aureus
Abstract
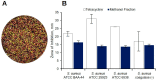
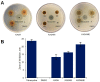
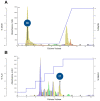
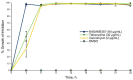
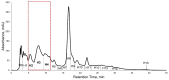
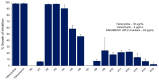
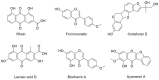
Cajanus cajan L. (pigeon pea, locally known in the Philippines as kadios) seed is a functional food with health benefits that extend beyond their nutritional value. C. cajan seeds contain highly diverse secondary metabolites with enriched beneficial properties, such as antibacterial, anticancer, and antioxidant activities. However, the antibacterial activities of secondary metabolites from Philippine-grown C. cajan, against multidrug-resistant Staphylococcus aureus have not been thoroughly described. Here, we investigated the in vitro antibacterial properties of C. cajan seed against multidrug-resistant S. aureus ATCC BAA-44 (MDRSA) and three other S. aureus strains (S. aureus ATCC 25923, S. aureus ATCC 6538, and coagulase-negative S. aureus) and, subsequently, identified the antibiotic markers against S. aureus strains using mass spectrometry. Secondary metabolites from C. cajan seeds were extracted using acetone, methanol, or 95% ethanol. Antibacterial screening revealed antibiotic activity for the C. cajan acetone extract. Bioassay-guided purification of the C. cajan acetone extract afforded three semi-pure high-performance liquid chromatography (HPLC) fractions exhibiting 32-64 µg/mL minimum inhibitory concentration (MIC) against MDRSA. Chemical profiling of these fractions using liquid chromatography mass spectrometry (LCMS) identified six compounds that are antibacterial against MDRSA. High-resolution mass spectrometry (HRMS), MS/MS, and dereplication using Global Natural Products Social Molecular Networking (GNPS)™, and National Institute of Standards and Technology (NIST) Library identified the metabolites as rhein, formononetin, laccaic acid D, crotafuran E, ayamenin A, and biochanin A. These isoflavonoids, anthraquinones, and pterocarpanoids from C. cajan seeds are potential bioactive compounds against S. aureus, including the multidrug-resistant strains.
Keywords: Cajanus cajan; anthraquinones; antibiotics; flavonoids; mass spectrometry; metabolomics; multidrug resistant Staphylococcus aureus; pigeon pea; pterocarpanoids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Isolation, purification and characterization of the antibacterial, antihypertensive and antioxidative properties of the bioactive peptides in the purified and proteolyzed major storage protein of pigeon pea (Cajanus cajan) seeds.Food Chem (Oxf). 2021 Dec 8;4:100062. doi: 10.1016/j.fochms.2021.100062. eCollection 2022 Jul 30. Food Chem (Oxf). 2021. PMID: 35415680 Free PMC article.
-
The Isorhamnetin-Containing Fraction of Philippine Honey Produced by the Stingless Bee Tetragonula biroi Is an Antibiotic against Multidrug-Resistant Staphylococcus aureus.Molecules. 2021 Mar 17;26(6):1688. doi: 10.3390/molecules26061688. Molecules. 2021. PMID: 33802916 Free PMC article.
-
GC-MS and LC-MS: an integrated approach towards the phytochemical evaluation of methanolic extract of Pigeon Pea [Cajanus cajan (L.) Millsp] leaves.Nat Prod Res. 2022 Apr;36(8):2177-2181. doi: 10.1080/14786419.2020.1849197. Epub 2020 Nov 23. Nat Prod Res. 2022. PMID: 33222530
-
Revisiting the Nutritional, Chemical and Biological Potential of Cajanus cajan (L.) Millsp.Molecules. 2022 Oct 13;27(20):6877. doi: 10.3390/molecules27206877. Molecules. 2022. PMID: 36296470 Free PMC article. Review.
-
In vitro activity of ceftaroline against multidrug-resistant Staphylococcus aureus and Streptococcus pneumoniae: a review of published studies and the AWARE Surveillance Program (2008-2010).Clin Infect Dis. 2012 Sep;55 Suppl 3:S206-14. doi: 10.1093/cid/cis563. Clin Infect Dis. 2012. PMID: 22903953 Review.
Cited by
-
Metabolome profile variations in common bean (Phaseolus vulgaris L.) resistant and susceptible genotypes incited by rust (Uromyces appendiculatus).Front Genet. 2023 Mar 16;14:1141201. doi: 10.3389/fgene.2023.1141201. eCollection 2023. Front Genet. 2023. PMID: 37007949 Free PMC article.
-
Plant antibacterials: The challenges and opportunities.Heliyon. 2024 May 11;10(10):e31145. doi: 10.1016/j.heliyon.2024.e31145. eCollection 2024 May 30. Heliyon. 2024. PMID: 38803958 Free PMC article. Review.
References
-
- World Health Organization. Worldwide Country Situation Analysis: Response to Antimicrobial Resistance. 2015. [(accessed on 31 July 2021)]. Available online: https://apps.who.int/iris/handle/10665/163473.
-
- World Health Organization. Antimicrobial Resistance Global Report on Surveillance. 2014. [(accessed on 31 July 2021)]. Available online: https://www.who.int/publications/i/item/9789241564748?msclkid=829eb582a8....
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

