Synthesis of Human Phase I and Phase II Metabolites of Hop (Humulus lupulus) Prenylated Flavonoids
- PMID: 35448532
- PMCID: PMC9030851
- DOI: 10.3390/metabo12040345
Synthesis of Human Phase I and Phase II Metabolites of Hop (Humulus lupulus) Prenylated Flavonoids
Abstract
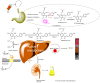
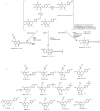
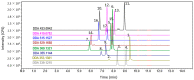
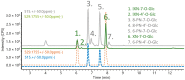
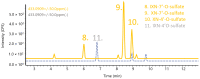
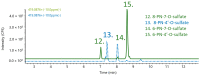
Hop prenylated flavonoids have been investigated for their in vivo activities due to their broad spectrum of positive health effects. Previous studies on the metabolism of xanthohumol using untargeted methods have found that it is first degraded into 8-prenylnaringenin and 6-prenylnaringenin, by spontaneous cyclisation into isoxanthohumol, and subsequently demethylated by gut bacteria. Further combinations of metabolism by hydroxylation, sulfation, and glucuronidation result in an unknown number of isomers. Most investigations involving the analysis of prenylated flavonoids used surrogate or untargeted approaches in metabolite identification, which is prone to errors in absolute identification. Here, we present a synthetic approach to obtaining reference standards for the identification of human xanthohumol metabolites. The synthesised metabolites were subsequently analysed by qTOF LC-MS/MS, and some were matched to a human blood sample obtained after the consumption of 43 mg of micellarised xanthohumol. Additionally, isomers of the reference standards were identified due to their having the same mass fragmentation pattern and different retention times. Overall, the methods unequivocally identified the metabolites of xanthohumol that are present in the blood circulatory system. Lastly, in vitro bioactive testing should be applied using metabolites and not original compounds, as free compounds are scarcely found in human blood.
Keywords: beer; blood analysis; hops; metabolites; prenylated flavonoids; synthesis; xanthohumol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Yang X., Jiang Y., Yang J., He J., Sun J., Chen F., Zhang M., Yang B. Prenylated flavonoids, promising nutraceuticals with impressive biological activities. Trends Food Sci. Technol. 2015;44:93–104. doi: 10.1016/j.tifs.2015.03.007. - DOI
-
- Awouafack M.D., Wong C.P., Tane P., Morita H. Prenylated Flavonoids in Food. In: Xiao J., Sarker S.D., Asakawa Y., editors. Handbook of Dietary Phytochemicals. Springer; Singapore: 2020. pp. 1–23.
-
- Butt M.S., Nazir A., Sultan M.T., Schroën K. Morus alba L. nature’s functional tonic. Trends Food Sci. Technol. 2008;19:505–512. doi: 10.1016/j.tifs.2008.06.002. - DOI
-
- Chang S.K., Jiang Y., Yang B. An update of prenylated phenolics: Food sources, chemistry and health benefits. Trends Food Sci. Technol. 2021;108:197–213. doi: 10.1016/j.tifs.2020.12.022. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

