Immunomodulating Activity of Pleurotus eryngii Mushrooms Following Their In Vitro Fermentation by Human Fecal Microbiota
- PMID: 35448559
- PMCID: PMC9028658
- DOI: 10.3390/jof8040329
Immunomodulating Activity of Pleurotus eryngii Mushrooms Following Their In Vitro Fermentation by Human Fecal Microbiota
Abstract
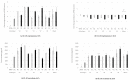
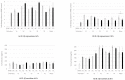
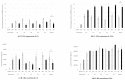
Recent studies have revealed the crucial role of several edible mushrooms and fungal compounds, mainly polysaccharides, in human health and disease. The investigation of the immunomodulating effects of mushroom polysaccharides, especially β-glucans, and the link between their anticancer and immunomodulatory properties with their possible prebiotic activity on gut micro-organisms has been the subject of intense research over the last decade. We investigated the immunomodulating effects of Pleurotus eryngii mushrooms, selected due to their high β-glucan content, strong lactogenic effect, and potent geno-protective properties, following in vitro fermentation by fecal inocula from healthy elderly volunteers (>60 years old). The immunomodulating properties of the fermentation supernatants (FSs) were initially investigated in U937-derived human macrophages. Gene expression as well as pro- (TNF-α, IL-1β) and anti-inflammatory cytokines (IL-10, IL-1Rα) were assessed and correlated with the fermentation process. The presence of P. eryngii in the fermentation process led to modifications in immune response, as indicated by the altered gene expression and levels of the cytokines examined, a finding consistent for all volunteers. The FSs immunomodulating effect on the volunteers’ peripheral blood mononuclear cells (PBMCs) was verified through the use of cytometry by time of flight (CyTOF) analysis.
Keywords: PBMCs; cytokines; edible mushrooms; macrophages.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Roncero-Ramos I., Delgado-Andrade C. The beneficial role of edible mushrooms in human health. Curr. Opin. Food Sci. 2017;14:122–128. doi: 10.1016/j.cofs.2017.04.002. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

