Combination of Haploidentical Hematopoietic Stem Cell Transplantation with Umbilical Cord-Derived Mesenchymal Stem Cells in Patients with Severe Aplastic Anemia: A Retrospective Controlled Study
- PMID: 35448935
- PMCID: PMC9160692
- DOI: 10.4274/tjh.galenos.2022.2022.0084
Combination of Haploidentical Hematopoietic Stem Cell Transplantation with Umbilical Cord-Derived Mesenchymal Stem Cells in Patients with Severe Aplastic Anemia: A Retrospective Controlled Study
Abstract
Objective: We retrospectively compared the outcomes of patients with severe aplastic anemia (SAA) who received haploidentical hematopoietic stem cell transplantation (haplo-HSCT) combined or not combined with umbilical cord-derived mesenchymal stem cells (UC-MSCs).
Materials and methods: A total of 101 patients with SAA were enrolled in this study and treated with haplo-HSCT plus UC-MSC infusion (MSC group, n=47) or haplo-HSCT alone (non-MSC group, n=54).
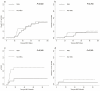
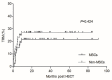
Results: The median time to neutrophil engraftment in the MSC and non-MSC group was 11 (range: 8-19) and 12 (range: 8-23) days, respectively (p=0.049), with a respective cumulative incidence (CI) of 97.82% and 97.96% (p=0.101). Compared to the non-MSC group, the MSC group had a lower CI of chronic graft-versus-host disease (GVHD) (8.60±0.25% vs. 24.57±0.48%, p=0.048), but similar rates of grades II-IV acute GVHD (23.40±0.39% vs. 24.49±0.39%, p=0.849), grades III-IV acute GVHD (8.51±0.17% vs. 10.20±0.19%, p=0.765), and moderate-severe chronic GVHD (2.38±0.06% vs. 7.45±0.18%, p=0.352) were observed. The estimated 5-year overall survival (OS) rates were 78.3±6.1% and 70.1±6.3% (p=0.292) while the estimated 5-year GVHD-free, failure-free survival (GFFS) rates were 76.6±6.2% and 56.7±6.9% (p=0.045) in the MSC and non-MSC groups, respectively.
Conclusion: In multivariate analysis, graft failure was the only adverse predictor for OS. Meanwhile, graft failure, grades III-IV acute GVHD, and moderate-severe chronic GVHD could predict worse GFFS. Our results indicated that haplo-HSCT combined with UC-MSCs infusion was an effective and safe option for SAA patients.
Amaç: Göbek kordonundan elde edilen mezenkimal kök hücrelerin (UK-MKH’ler) infüzyonu ile kombine edilmiş ve edilmemiş, haploidentik hematopoietik kök hücre nakli (haplo-HKHN) yapılan şiddetli aplastik anemili (ŞAA) hastalarının sonuçları geriye dönük olarak karşılaştırıldı.
Gereç ve yöntemler: Bu çalışmaya ŞAA’lı toplam 101 hasta alındı ve hastalar haplo-HSCT ile birlikte UK-MKH infüzyonu (MKH grubu, N=47) ve tek başına haplo-HKHN (MKH olmayan grup, N=54) şeklinde iki gruba ayrıldı.
Bulgular: MKH alan ve almayan gruplarda nötrofil engraftmanı için medyan süreler sırasıyla, 11 (aralık, 8-19) ve 12 (aralık, 8-23) gün (p=0,049) ve kümülatif insidans (CI) sırası ile %97,82 ve %97,96 (p=0,101) bulundu. MKH almayan grupla karşılaştırıldığında, MKH alan grupta daha düşük kronik graft-versus-host hastalığı (cGVHD) CI saptandı (%8,60±0,25’e karşı %24,57±0,48, p=0,048), ancak II-IV akut GVHD (aGVHD) (%23,40±0,39-%24,49±0,39, p=0,849), III-IV aGVHD (%8,51±0,17 ve %10,20±0,19, p=0,765) ve orta-şiddetli cGVHD (%2,38±0,06 vs. %7,45±0,18, p=0,352) gruplarında fark görülmedi. MKH alan ve MKH almayan gruplarda tahmini beş yıllık genel sağkalım oranları %78,3±6,1 ve %70,1±6,3 (p=0,292) iken, GVHD’siz, hastalıksız sağkalım (GFFS) oranları sırasıyla, %76,6 ±%6,2 ve %56,7±6,9 (p=0,045) idi.
Sonuç: Çok değişkenli analizde greft yetmezliği, OS için tek olumsuz gösterge idi. Bu arada, greft yetmezliği, III-IV aGVHD ve orta-şiddetli cGVHD arasında daha kötü GFFS’yi öngörebilir. Sonuçlarımız, UC-MKH infüzyonu ile birlikte haplo-HKHN’nin ŞAA hastaları için etkili ve güvenli bir seçenek olduğunu gösterdi.
Keywords: Haploidentical; Severe aplastic anemia; Hematopoieticstem cell transplantation; Mesenchymal stem cells.
Conflict of interest statement
Figures

References
-
- Zhu XF, He HL, Wang SQ, Tang JY, Han B, Zhang DH, Wu LQ, Wu DP, Li W, Xia LH, Zhu HL, Liu F, Shi HX, Zhang X, Zhou F, Hu JD, Fang JP, Chen XQ, Ye TZ, Liang YM, Jin J, Zhang FK. Current treatment patterns of aplastic anemia in China: a prospective cohort registry study. Acta Haematol. 2019;142:162–170. - PMC - PubMed
-
- ElGohary G, El Fakih R, de Latour R, Risitano A, Marsh J, Schrezenmeier H, Gluckman E, Hochsmann B, Pierri F, Halkes C, Alzahrani H, De la Fuente J, Cesaro S, Alahmari A, Ahmed SO, Passweg J, Dufour C, Bacigalupo A, Aljurf M. Haploidentical hematopoietic stem cell transplantation in aplastic anemia: a systematic review and meta-analysis of clinical outcome on behalf of the severe aplastic anemia working party of the European group for blood and marrow transplantation (SAAWP of EBMT) Bone Marrow Transplant. 2020;55:1906–1917. - PubMed
-
- Killick SB, Bown N, Cavenagh J, Dokal I, Foukaneli T, Hill A, Hillmen P, Ireland R, Kulasekararaj A, Mufti G, Snowden JA, Samarasinghe S, Wood A, Marsh JC; British Society for Standards in Haematology. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172:187–207. - PubMed
-
- Bacigalupo A, Giammarco S, Sica S. Bone marrow transplantation versus immunosuppressive therapy in patients with acquired severe aplastic anemia. Int J Hematol. 2016;104:168–174. - PubMed
-
- Xu LP, Zhang XH, Wang FR, Mo XD, Han TT, Han W, Chen YH, Zhang YY, Wang JZ, Yan CH, Sun YQ, Zuo SN, Huang XJ. Haploidentical transplantation for pediatric patients with acquired severe aplastic anemia. Bone Marrow Transplant. 2017;52:381–387. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
