Bayesian adaptive design for pediatric clinical trials incorporating a community of prior beliefs
- PMID: 35448963
- PMCID: PMC9027907
- DOI: 10.1186/s12874-022-01569-x
Bayesian adaptive design for pediatric clinical trials incorporating a community of prior beliefs
Abstract
Background: Pediatric population presents several barriers for clinical trial design and analysis, including ethical constraints on the sample size and slow accrual rate. Bayesian adaptive design methods could be considered to address these challenges in pediatric clinical trials.
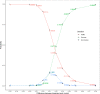
Methods: We developed an innovative Bayesian adaptive design method and demonstrated the approach as a re-design of a published phase III pediatric trial. The innovative design used early success criteria based on skeptical prior and early futility criteria based on enthusiastic prior extrapolated from a historical adult trial, and the early and late stopping boundaries were calibrated to ensure a one-sided type I error of 2.5%. We also constructed several alternative designs which incorporated only one type of prior belief and the same stopping boundaries. To identify a preferred design, we compared operating characteristics including power, expected trial size and trial duration for all the candidate adaptive designs via simulation when performing an increasing number of equally spaced interim analyses.
Results: When performing an increasing number of equally spaced interim analyses, the innovative Bayesian adaptive trial design incorporating both skeptical and enthusiastic priors at both interim and final analyses outperforms alternative designs which only consider one type of prior belief, because it allows more reduction in sample size and trial duration while still offering good trial design properties including controlled type I error rate and sufficient power.
Conclusions: Designing a Bayesian adaptive pediatric trial with both skeptical and enthusiastic priors can be an efficient and robust approach for early trial stopping, thus potentially saving time and money for trial conduction.
Keywords: Bayesian adaptive design; Interim analysis; Pediatric clinical trials; Prior belief.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- EMA. ICH E11(R1) guideline on clinical investigation of medicinal products in the pediatric population. 2017 [Cited 2021 April 21]; Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e11r1-gu...
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

