Oxidative stress generated by polycyclic aromatic hydrocarbons from ambient particulate matter enhance vascular smooth muscle cell migration through MMP upregulation and actin reorganization
- PMID: 35449013
- PMCID: PMC9026692
- DOI: 10.1186/s12989-022-00472-z
Oxidative stress generated by polycyclic aromatic hydrocarbons from ambient particulate matter enhance vascular smooth muscle cell migration through MMP upregulation and actin reorganization
Abstract
Background: Epidemiological studies have suggested that elevated concentrations of particulate matter (PM) are strongly associated with the incidence of atherosclerosis, however, the underlying cellular and molecular mechanisms of atherosclerosis by PM exposure and the components that are mainly responsible for this adverse effect remain to be established. In this investigation, we evaluated the effects of ambient PM on vascular smooth muscle cell (VSMC) behavior. Furthermore, the effects of polycyclic aromatic hydrocarbons (PAHs), major components of PM, on VSMC migration and the underlying mechanisms were examined.
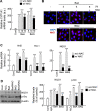
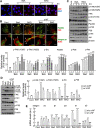
Results: VSMC migration was significantly increased by treatment with organic matters extracted from ambient PM. The total amount of PAHs contained in WPM was higher than that in SPM, leading to higher ROS generation and VSMC migration. The increased migration was successfully inhibited by treatment with the anti-oxidant, N-acetyl-cysteine (NAC). The levels of matrix metalloproteinase (MMP) 2 and 9 were significantly increased in ambient PM-treated VSMCs, with MMP9 levels being significantly higher in WPM-treated VSMCs than in those treated with SPM. As expected, migration was significantly increased in all tested PAHs (anthracene, ANT; benz(a)anthracene, BaA) and their oxygenated derivatives (9,10-Anthraquinone, AQ; 7,12-benz(a)anthraquinone, BAQ, respectively). The phosphorylated levels of focal adhesion kinase (FAK) and formation of the focal adhesion complex were significantly increased in ambient PM or PAH-treated VSMCs, and these effects were blocked by administration of NAC or α-NF, an inhibitor of AhR, the receptor that allows PAH uptake. Subsequently, the levels of phosphorylated Src and NRF, the downstream targets of FAK, were altered with a pattern similar to that of p-FAK.
Conclusions: PAHs, including oxy-PAHs, in ambient PM may have dual effects that lead to an increase in VSMC migration. One is the generation of oxidative stress followed by MMP upregulation, and the other is actin reorganization that results from the activation of the focal adhesion complex.
Keywords: Ambient particulate matter; Matrix metalloproteinase; Migration; Oxidative stress; Polycyclic aromatic hydrocarbons; Vascular smooth muscle cells.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Jilani MH, Simon-Friedt B, Yahya T, Khan AY, Hassan SZ, Kash B, et al. Associations between particulate matter air pollution, presence and progression of subclinical coronary and carotid atherosclerosis: a systematic review. Atherosclerosis. 2020;306:22–32. doi: 10.1016/j.atherosclerosis.2020.06.018. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

