Identifying clinical subtypes in sepsis-survivors with different one-year outcomes: a secondary latent class analysis of the FROG-ICU cohort
- PMID: 35449071
- PMCID: PMC9022336
- DOI: 10.1186/s13054-022-03972-8
Identifying clinical subtypes in sepsis-survivors with different one-year outcomes: a secondary latent class analysis of the FROG-ICU cohort
Abstract
Background: Late mortality risk in sepsis-survivors persists for years with high readmission rates and low quality of life. The present study seeks to link the clinical sepsis-survivors heterogeneity with distinct biological profiles at ICU discharge and late adverse events using an unsupervised analysis.
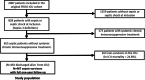
Methods: In the original FROG-ICU prospective, observational, multicenter study, intensive care unit (ICU) patients with sepsis on admission (Sepsis-3) were identified (N = 655). Among them, 467 were discharged alive from the ICU and included in the current study. Latent class analysis was applied to identify distinct sepsis-survivors clinical classes using readily available data at ICU discharge. The primary endpoint was one-year mortality after ICU discharge.
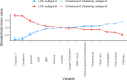
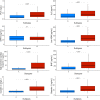
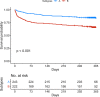
Results: At ICU discharge, two distinct subtypes were identified (A and B) using 15 readily available clinical and biological variables. Patients assigned to subtype B (48% of the studied population) had more impaired cardiovascular and kidney functions, hematological disorders and inflammation at ICU discharge than subtype A. Sepsis-survivors in subtype B had significantly higher one-year mortality compared to subtype A (respectively, 34% vs 16%, p < 0.001). When adjusted for standard long-term risk factors (e.g., age, comorbidities, severity of illness, renal function and duration of ICU stay), subtype B was independently associated with increased one-year mortality (adjusted hazard ratio (HR) = 1.74 (95% CI 1.16-2.60); p = 0.006).
Conclusions: A subtype with sustained organ failure and inflammation at ICU discharge can be identified from routine clinical and laboratory data and is independently associated with poor long-term outcome in sepsis-survivors. Trial registration NCT01367093; https://clinicaltrials.gov/ct2/show/NCT01367093 .
Keywords: Biomarkers; Latent profile analysis; Mixture modeling; Personalized medicine; Post-intensive care syndrome (PICS); Prognostic enrichment; Sepsis.
© 2022. The Author(s).
Conflict of interest statement
None of the authors of this paper has a financial or personal relationship with other persons or organizations that could inappropriately influence or bias the content of the paper. Dr. P.J has received honoraria to the institution for participation in advisory boards from Amgen; has received research grants to the institution from AstraZeneca (Cambridge, United Kingdom), Biotronik (Berlin, Germany), Biosensors International (Singapore), Eli Lilly (Indianapolis, Indiana) and the Medicines Company (Parsippany-Troy Hills, New Jersey); and serves as an unpaid member of the steering groups of trials funded by AstraZeneca (Cambridge, United Kingdom), Biotronik (Berlin, Germany), Biosensors (Singapore), St. Jude Medical (St. Paul, Minnesota) and the Medicines Company. Dr. L.B. laboratory reports grants from Medtronic Covidien, Draeger, non-financial support from Fisher Paykel, non-financial support from Philips, non-financial support from Sentec, non-financial support from Air Liquide, a patent with General Electric, outside the submitted work. Dr. P.R.L. has received unrelated research funding from the Canadian Institutes of Health Research, the U.S. National Institutes of Health (National Heart, Lung, and Blood Institute), the Peter Munk Cardiac Centre, the LifeArc Foundation, the Thistledown Foundation, the Ted Rogers Centre for Heart Research, the Medicine by Design Fund, the University of Toronto and the Government of Ontario. He also received unrelated consulting honoraria from Novartis, Corrona and Brigham and Women’s Hospital, as well as unrelated royalties from McGraw-Hill Publishing. Dr. A.M. has received speaker’s honoraria from Abbott (Chicago, Illinois), Orion (Auckland, New Zealand), Roche (Basel, Switzerland) and Servier (Suresnes, France); and fees as a member of the advisory boards and/or steering committees and/or research grants from BMS (New York, New York), Adrenomed (Hennigsdorf, Germany), Neurotronik (Durham, North Carolina), Roche (Basel, Switzerland), Sanofi (Paris, France), Sphyngotec (Hennigsdorf, Germany), Novartis (Basel, Switzerland), Otsuka (Chiyoda City, Tokyo, Japan), Philips (Amsterdam, Netherlands) and 4TEEN4 (Hennigsdorf, Germany). Dr. E.G. received fees as a member of the advisory boards and/or steering committees and/or from research grants from Magnisense (Paris, France), Adrenomed (Hennigsdorf, Germany) and Deltex Medical (Chichester, United Kingdom). The remaining authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

