Hsa-miR-30a-3p overcomes the acquired protective autophagy of bladder cancer in chemotherapy and suppresses tumor growth and muscle invasion
- PMID: 35449123
- PMCID: PMC9023440
- DOI: 10.1038/s41419-022-04791-z
Hsa-miR-30a-3p overcomes the acquired protective autophagy of bladder cancer in chemotherapy and suppresses tumor growth and muscle invasion
Erratum in
-
Correction: Hsa-miR-30a-3p overcomes the acquired protective autophagy of bladder cancer in chemotherapy and suppresses tumor growth and muscle invasion.Cell Death Dis. 2022 May 31;13(5):514. doi: 10.1038/s41419-022-04889-4. Cell Death Dis. 2022. PMID: 35641485 Free PMC article. No abstract available.
Abstract
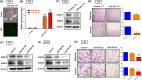
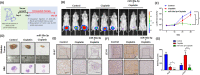
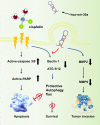
Bladder cancer (BC) is the second most common urologic cancer in western countries. New strategies for managing high-grade muscle-invasive bladder cancer (MIBC) are urgently required because MIBC has a high risk of recurrence and poor survival. A growing body of evidence indicates that microRNA has potent antitumorigenic properties in various cancers, and thus, therapeutic strategies based on microRNA may show promising results in cancer therapy. Analysis of The Cancer Genome Atlas (TCGA) database indicated that hsa-miR-30a-3p is downregulated in human BC. Our in vitro investigation demonstrated that hsa-miR-30a-3p suppresses the expression of matrix metalloproteinase-2 (MMP-2) and MMP-9 and reduces the cell invasive potential of BC cells. Furthermore, hsa-miR-30a-3p directly targets ATG5, ATG12, and Beclin 1; this in turn improves the chemosensitivity of BC cells to cisplatin through the repression of protective autophagy. In a tumor-xenograft mice model, hsa-miR-30a-3p suppressed muscle invasion. Cotreatment with hsa-miR-30a-3p enhanced the antitumor effect of cisplatin in reducing tumor growth in BC. The current study provides a novel strategy of using hsa-miR-30a-3p as an adjuvant or replacement therapy in future BC treatment.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Sanli O, Dobruch J, Knowles MA, Burger M, Alemozaffar M, Nielsen ME, et al. Bladder cancer. Nat Rev Dis Prim. 2017;3:17022. - PubMed
-
- Kamat AM, Hahn NM, Efstathiou JA, Lerner SP, Malmstrom PU, Choi W, et al. Bladder cancer. Lancet. 2016;388:2796–810. - PubMed
-
- Rosario DJ, Becker M, Anderson JB. The changing pattern of mortality and morbidity from radical cystectomy. BJU Int. 2000;85:427–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

