Proton pump inhibitors affect capecitabine efficacy in patients with stage II-III colorectal cancer: a multicenter retrospective study
- PMID: 35449143
- PMCID: PMC9023444
- DOI: 10.1038/s41598-022-10008-2
Proton pump inhibitors affect capecitabine efficacy in patients with stage II-III colorectal cancer: a multicenter retrospective study
Abstract
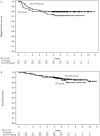
The association between capecitabine efficacy and proton pump inhibitors (PPIs) is controversial. Here, we determined whether co-administration of PPIs affects the real-world effectiveness of capecitabine. This retrospective observational study included consecutive patients with stage II-III colorectal cancer (CRC) who received adjuvant capecitabine monotherapy or CapeOX (capecitabine and oxaliplatin) between January 2009 and December 2014 at nine participating institutions. The primary endpoint was the difference in relapse-free survival (RFS) between patients who received PPIs and those who did not and was estimated using the Kaplan-Meier method. Overall survival (OS) was the secondary endpoint. Multivariable analysis of RFS and OS was performed using a Cox proportional hazards model, propensity score adjustment, and inverse probability of treatment weighting (IPTW) analyses. Data from 606 patients were evaluated, 54 of whom had received a PPI. PPI-treated patients tended to have poorer RFS and OS than patients treated without PPIs. The hazard ratio for RFS with capecitabine monotherapy was 2.48 (95% confidence interval: 1.22-5.07). These results were consistent with sensitivity analyses performed using propensity score adjustment and IPTW methods. Co-administration of PPIs may reduce the effectiveness of capecitabine and negatively impact patients with stage II-III CRC.
© 2022. The Author(s).
Conflict of interest statement
R.U. received personal fees from Eisai, Sawai Pharmaceutical, and CAC Croit outside the submitted work. Other authors have no conflicts of interest to disclose.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

