Establishment of a fish model to study gas-bubble lesions
- PMID: 35449183
- PMCID: PMC9023494
- DOI: 10.1038/s41598-022-10539-8
Establishment of a fish model to study gas-bubble lesions
Erratum in
-
Author Correction: Establishment of a fish model to study gas-bubble lesions.Sci Rep. 2024 Jan 30;14(1):2528. doi: 10.1038/s41598-024-52889-5. Sci Rep. 2024. PMID: 38291085 Free PMC article. No abstract available.
Abstract
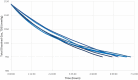
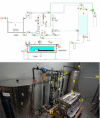
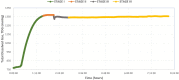
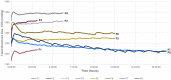
Decompression sickness (DCS) is a clinical syndrome caused by the formation of systemic intravascular and extravascular gas bubbles. The presence of these bubbles in blood vessels is known as gas embolism. DCS has been described in humans and animals such as sea turtles and cetaceans. To delve deeper into DCS, experimental models in terrestrial mammals subjected to compression/decompression in a hyperbaric chamber have been used. Fish can suffer from gas bubble disease (GBD), characterized by the formation of intravascular and extravascular systemic gas bubbles, similarly to that observed in DCS. Given these similarities and the fact that fish develop this disease naturally in supersaturated water, they could be used as an alternative experimental model for the study of the pathophysiological aspect of gas bubbles. The objective of this study was to obtain a reproducible model for GBD in fish by an engineering system and a complete pathological study, validating this model for the study of the physiopathology of gas related lesions in DCS. A massive and severe GBD was achieved by exposing the fish for 18 h to TDG values of 162-163%, characterized by the presence of severe hemorrhages and the visualization of massive quantities of macroscopic and microscopic gas bubbles, systemically distributed, circulating through different large vessels of experimental fish. These pathological findings were the same as those described in small mammals for the study of explosive DCS by hyperbaric chamber, validating the translational usefulness of this first fish model to study the gas-bubbles lesions associated to DCS from a pathological standpoint.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Edmonds C, Bennett M, Lippmann J, Mitchell S. Diving and Subaquatic Medicine. CRC Press; 2015.
-
- Bert P. LaPressionBarometrique:RecherchesdePhysiologieExpérimentale. Masson; 1878.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

