Transcutaneous electrical acupoint stimulation for children with attention-deficit/hyperactivity disorder: a randomized clinical trial
- PMID: 35449191
- PMCID: PMC9022403
- DOI: 10.1038/s41398-022-01914-0
Transcutaneous electrical acupoint stimulation for children with attention-deficit/hyperactivity disorder: a randomized clinical trial
Abstract
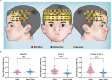
Little is known about the effects of transcutaneous electrical acupoint stimulation (TEAS) for children with attention-deficit/hyperactivity disorder (ADHD). Here, we carried out a 4 week randomized clinical trial in which patients aged 6-12 years old with an ADHD diagnosis received TEAS or sham TEAS. The primary outcome measure was the investigator-rated Clinical Global Impression-Improvement (CGI-I) score at week 4. Secondary outcomes included changes from baseline to week 4 in the investigator-rated Clinical Global Impression-Severity of Illness (CGI-S) score, the Conners' Parent/Teacher Rating Scales-Revised: Short Form (CPRS-R: S/CTRS-R: S) score, go/no-go task performance, and functional near-infrared spectroscopy (fNIRS)-based oxygenated hemoglobin level within the prefrontal cortex. At week 4, the CGI-I score indicated improvement in 33.3% of the TEAS group compared with 7.7% of the sham group (P = 0.005). The TEAS group had a greater decrease in the mean CGI-S score (-0.87) than the sham TEAS group (-0.28) (P = 0.003). A greater enhancement in the mean cerebral oxygenated hemoglobin within the prefrontal cortex was found in the TEAS group (0.099 mM mm) compared with the sham TEAS group (0.005 mM mm) (P < 0.001). CPRS-R: S score, CTRS-R: S score, and go/no-go performance exhibited no significant improvement after TEAS treatment. The manipulation-associated adverse events were uncommon in both groups, and events were very mild. Our results show that noninvasive TEAS significantly improved general symptoms and increased prefrontal cortex blood flow within 4 weeks for children with ADHD. Further clinical trials are required to understand the long-term efficacy in a larger clinical sample. This trial was registered on ClinicalTrials.gov (NCT03917953).
© 2022. The Author(s).
Conflict of interest statement
LAR has received grant or research support from, served as a consultant to, and served on the speakers’ bureau of Bial, Medice, Novartis/Sandoz, Pfizer and Shire/Takeda in the last 3 years. The ADHD and Juvenile Bipolar Disorder Outpatient Programs chaired by LAR have received unrestricted educational and research support from the following pharmaceutical companies in the last 3 years: Novartis/Sandoz and Shire/Takeda. LAR has received authorship royalties from Oxford Press and ArtMed and travel grants from Shire to take part in the 2018 APA annual meeting. All other authors declare no competing interests.
Figures
Similar articles
-
The usefulness of Conners' Rating Scales-Revised in screening for attention deficit hyperactivity disorder in children with intellectual disabilities and borderline intelligence.J Intellect Disabil Res. 2008 Nov;52(11):950-65. doi: 10.1111/j.1365-2788.2007.01035.x. Epub 2008 Jan 2. J Intellect Disabil Res. 2008. PMID: 18179511
-
A randomized double-blind study of atomoxetine versus placebo for attention-deficit/hyperactivity disorder symptoms in children with autism spectrum disorder.J Am Acad Child Adolesc Psychiatry. 2012 Jul;51(7):733-41. doi: 10.1016/j.jaac.2012.04.011. Epub 2012 May 25. J Am Acad Child Adolesc Psychiatry. 2012. PMID: 22721596 Clinical Trial.
-
Transcutaneous electrical acupoint stimulation for cancer-related pain management in patients receiving chronic opioid therapy: a randomized clinical trial.Support Care Cancer. 2023 Dec 12;32(1):16. doi: 10.1007/s00520-023-08240-1. Support Care Cancer. 2023. PMID: 38085376 Clinical Trial.
-
[Atomoxetine: a new treatment for Attention Deficit/Hyperactivity Disorder (ADHD) in children and adolescents].Encephale. 2005 May-Jun;31(3):337-48. doi: 10.1016/s0013-7006(05)82399-1. Encephale. 2005. PMID: 16142049 Review. French.
-
Efficacy and Safety of Transcutaneous Electrical Acupoint Stimulation (TEAS) As An Analgesic Intervention for Labor Pain: A Network Meta-analysis of Randomized Controlled Trials.Pain Ther. 2023 Jun;12(3):631-644. doi: 10.1007/s40122-023-00496-z. Epub 2023 Mar 19. Pain Ther. 2023. PMID: 36934401 Free PMC article. Review.
Cited by
-
Effect of transcutaneous electrical acupoint stimulation on pregnancy outcomes in women with in vitro fertilization-embryo transfer: A systematic review and meta-analysis.Front Cell Dev Biol. 2022 Dec 12;10:1068894. doi: 10.3389/fcell.2022.1068894. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36578784 Free PMC article. Review.
-
Vitamin D insufficiency and sleep disturbances in children with ADHD: a case-control study.Front Psychiatry. 2025 Mar 20;16:1546692. doi: 10.3389/fpsyt.2025.1546692. eCollection 2025. Front Psychiatry. 2025. PMID: 40182203 Free PMC article.
-
Aberrant amplitude of low-frequency fluctuation and functional connectivity in children with different subtypes of ADHD: a resting-state fNIRS study.BMC Psychiatry. 2024 Dec 18;24(1):919. doi: 10.1186/s12888-024-06350-6. BMC Psychiatry. 2024. PMID: 39696119 Free PMC article.
-
Effect of Different Frequencies of Transcutaneous Electrical Acupoint Stimulation (TEAS) on EEG Source Localization in Healthy Volunteers: A Semi-Randomized, Placebo-Controlled, Crossover Study.Brain Sci. 2025 Mar 3;15(3):270. doi: 10.3390/brainsci15030270. Brain Sci. 2025. PMID: 40149791 Free PMC article.
-
Transcutaneous electrical acupoint stimulation alleviates insomnia, negative emotions, and neurotransmitter imbalance in methamphetamine withdrawal: A randomized controlled trial.Medicine (Baltimore). 2025 Aug 1;104(31):e43508. doi: 10.1097/MD.0000000000043508. Medicine (Baltimore). 2025. PMID: 40760644 Free PMC article. Clinical Trial.
References
-
- Park S, Kim BN, Cho SC, Kim JW, Shin MS, Yoo HJ, et al. Baseline severity of parent-perceived inattentiveness is predictive of the difference between subjective and objective methylphenidate responses in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2013;23:410–4. doi: 10.1089/cap.2013.0031. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

