The Durable Safety and Effectiveness of Lixisenatide in Japanese People with Type 2 Diabetes: The Post-Marketing Surveillance PRANDIAL Study
- PMID: 35449321
- PMCID: PMC9122860
- DOI: 10.1007/s12325-022-02121-5
The Durable Safety and Effectiveness of Lixisenatide in Japanese People with Type 2 Diabetes: The Post-Marketing Surveillance PRANDIAL Study
Abstract
Introduction: Real-world evidence on lixisenatide in Japanese people with type 2 diabetes (T2D) is lacking. Therefore, the 3-year post-marketing PRANDIAL study was conducted to evaluate the safety (primary objective) and effectiveness (secondary objective) of lixisenatide in Japanese people with T2D during routine clinical practice.
Methods: This prospective, observational, multicenter, open-label study was conducted in Japanese individuals with T2D who initiated lixisenatide treatment between March 2014 and June 2017. Using electronic case report forms, investigators collected baseline demographic and clinical information and data on medications, safety and effectiveness up to 3 years after initiation of lixisenatide.
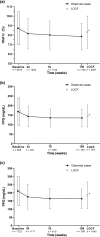
Results: Overall, 3046 participants were analyzed; their mean ± standard deviation (SD) age was 58.9 ± 13.1 years, and 53.7% were male. Mean ± SD duration of T2D was 12.8 ± 8.6 years, and baseline glycated hemoglobin (HbA1c) was 8.7% ± 1.7%. Most participants (93.9%) were receiving concomitant antidiabetic medications when they initiated lixisenatide. Median (range) lixisenatide treatment duration was 382 (1-1096) days. Adverse drug reactions (ADRs) were reported in 604 participants (19.8%) and serious ADRs in 22 (0.7%). The most common ADR was nausea (9.0%). Of ADRs of special interest, hypoglycemia occurred in 2.9% of participants, injection site reactions in 0.9%, and hypoglycemic unconsciousness in 0.03%. Baseline characteristics associated with an increased risk of ADRs (p < 0.05) were history of treatment for cardiovascular disease, hepatic dysfunction, and other complications. Effectiveness was analyzed in 2675 participants; HbA1c, fasting plasma glucose, postprandial glucose, and body weight all decreased significantly at last observation (all p < 0.0001 vs. baseline).
Conclusions: Lixisenatide was well tolerated, with no unexpected ADRs or new safety signals identified, and showed effective glycemic control and weight reduction up to 3 years, supporting the use of lixisenatide as a safe and effective treatment option for T2D in routine clinical practice in Japan.
Keywords: Japan; Lixisenatide; PRANDIAL study; Post-marketing surveillance; Type 2 diabetes.
Plain language summary
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are antidiabetic drugs that lower blood glucose levels by stimulating the release of insulin and suppressing glucagon, the key hormones involved in controlling blood glucose levels in the body. The selective GLP-1RA lixisenatide was approved for the management of adults with type 2 diabetes (T2D) in Japan based on data from randomized clinical trials. However, these studies may not be representative of the safety and effectiveness of the drug when used in routine clinical practice. Therefore, we conducted the 3-year post-marketing PRANDIAL study to assess the safety and effectiveness of lixisenatide in 3046 Japanese individuals with T2D who started the drug between March 2014 and June 2017. Adverse drug reactions (adverse events for which lixisenatide causality could not be excluded) occurred in 19.8% of participants, with the most common adverse drug reaction being nausea. Hypoglycemia (abnormally low blood glucose levels) was reported in 2.9%. Individuals with a history of treatment for cardiovascular disease, hepatic dysfunction, and other complications had an increased risk of adverse drug reactions. Lixisenatide provided significant improvements in blood glucose control, with significant decreases in glycated hemoglobin (a marker of blood glucose control), fasting plasma glucose, and postprandial glucose levels from baseline, as well as significant reductions in body weight. In this real-world post-marketing surveillance study, lixisenatide was well tolerated, raising no new safety concerns, and provided durable effective blood glucose control and weight reduction. These results support the use of lixisenatide in Japanese individuals with T2D in routine clinical practice.
© 2022. The Author(s).
Figures

Similar articles
-
Simultaneous Versus Sequential Initiation of Lixisenatide and Basal Insulin for Type 2 Diabetes: Subgroup Analysis of a Japanese Post-Marketing Surveillance Study of Lixisenatide (PRANDIAL).Adv Ther. 2022 Dec;39(12):5453-5473. doi: 10.1007/s12325-022-02311-1. Epub 2022 Oct 7. Adv Ther. 2022. PMID: 36207508 Free PMC article.
-
Real-World Safety and Effectiveness of Canagliflozin Treatment for Type 2 Diabetes Mellitus in Japan: SAPPHIRE, a Long-Term, Large-Scale Post-Marketing Surveillance.Adv Ther. 2022 Jan;39(1):674-691. doi: 10.1007/s12325-021-01984-4. Epub 2021 Dec 2. Adv Ther. 2022. PMID: 34853985 Free PMC article.
-
Safety of iGlarLixi in Japanese People with Type 2 Diabetes: A Post-marketing Database Study.Adv Ther. 2025 May;42(5):2168-2189. doi: 10.1007/s12325-025-03135-5. Epub 2025 Mar 8. Adv Ther. 2025. PMID: 40056372 Free PMC article.
-
The design and discovery of lixisenatide for the treatment of type 2 diabetes mellitus.Expert Opin Drug Discov. 2014 Oct;9(10):1223-51. doi: 10.1517/17460441.2014.942638. Epub 2014 Aug 14. Expert Opin Drug Discov. 2014. PMID: 25119443 Review.
-
Lixisenatide: A New Daily GLP-1 Agonist for Type 2 Diabetes Management.Ann Pharmacother. 2017 May;51(5):401-409. doi: 10.1177/1060028017689878. Epub 2017 Jan 29. Ann Pharmacother. 2017. PMID: 28133970 Review.
Cited by
-
Simultaneous Versus Sequential Initiation of Lixisenatide and Basal Insulin for Type 2 Diabetes: Subgroup Analysis of a Japanese Post-Marketing Surveillance Study of Lixisenatide (PRANDIAL).Adv Ther. 2022 Dec;39(12):5453-5473. doi: 10.1007/s12325-022-02311-1. Epub 2022 Oct 7. Adv Ther. 2022. PMID: 36207508 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

