CircACTR2 in macrophages promotes renal fibrosis by activating macrophage inflammation and epithelial-mesenchymal transition of renal tubular epithelial cells
- PMID: 35449370
- PMCID: PMC11072867
- DOI: 10.1007/s00018-022-04247-9
CircACTR2 in macrophages promotes renal fibrosis by activating macrophage inflammation and epithelial-mesenchymal transition of renal tubular epithelial cells
Abstract
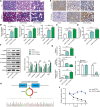
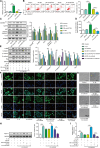
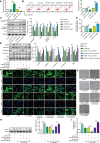
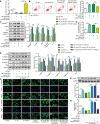
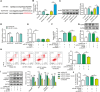
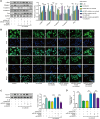
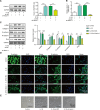
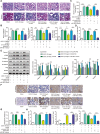
The crosstalk between macrophages and tubular epithelial cells (TECs) actively regulates the progression of renal fibrosis. In the present study, we revealed the significance of circular RNA ACTR2 (circACTR2) in regulating macrophage inflammation, epithelial-mesenchymal transition (EMT) of TECs, and the development of renal fibrosis. Our results showed UUO-induced renal fibrosis was associated with increased inflammation and EMT, hypertrophy of contralateral kidney, up-regulations of circACTR2 and NLRP3, and the down-regulation of miR-561. CircACTR2 sufficiently and essentially promoted the activation of NLRP3 inflammasome, pyroptosis, and inflammation in macrophages, and through paracrine effect, stimulated EMT and fibrosis of TECs. Mechanistically, circACTR2 sponged miR-561 and up-regulated NLRP3 expression level to induce the secretion of IL-1β. In TECs, IL-1β induced renal fibrosis via up-regulating fascin-1. Knocking down circACTR2 or elevating miR-561 potently alleviated renal fibrosis in vivo. In summary, circACTR2, by sponging miR-561, activated NLRP3 inflammasome, promoted macrophage inflammation, and stimulated macrophage-induced EMT and fibrosis of TECs. Knocking down circACTR2 and overexpressing miR-561 may, thus, benefit the treatment of renal fibrosis.
Keywords: Epithelial–mesenchymal transition; Fascin-1; Macrophages inflammation; NLRP3; Renal fibrosis; circACTR2; miR-561.
© 2022. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

