Optical coherence tomography in coronary atherosclerosis assessment and intervention
- PMID: 35449407
- PMCID: PMC9982688
- DOI: 10.1038/s41569-022-00687-9
Optical coherence tomography in coronary atherosclerosis assessment and intervention
Erratum in
-
Author Correction: Optical coherence tomography in coronary atherosclerosis assessment and intervention.Nat Rev Cardiol. 2024 May;21(5):348. doi: 10.1038/s41569-023-00982-z. Nat Rev Cardiol. 2024. PMID: 38110566 Free PMC article. No abstract available.
Abstract
Since optical coherence tomography (OCT) was first performed in humans two decades ago, this imaging modality has been widely adopted in research on coronary atherosclerosis and adopted clinically for the optimization of percutaneous coronary intervention. In the past 10 years, substantial advances have been made in the understanding of in vivo vascular biology using OCT. Identification by OCT of culprit plaque pathology could potentially lead to a major shift in the management of patients with acute coronary syndromes. Detection by OCT of healed coronary plaque has been important in our understanding of the mechanisms involved in plaque destabilization and healing with the rapid progression of atherosclerosis. Accurate detection by OCT of sequelae from percutaneous coronary interventions that might be missed by angiography could improve clinical outcomes. In addition, OCT has become an essential diagnostic modality for myocardial infarction with non-obstructive coronary arteries. Insight into neoatherosclerosis from OCT could improve our understanding of the mechanisms of very late stent thrombosis. The appropriate use of OCT depends on accurate interpretation and understanding of the clinical significance of OCT findings. In this Review, we summarize the state of the art in cardiac OCT and facilitate the uniform use of this modality in coronary atherosclerosis. Contributions have been made by clinicians and investigators worldwide with extensive experience in OCT, with the aim that this document will serve as a standard reference for future research and clinical application.
© 2022. Springer Nature Limited.
Conflict of interest statement
Disclosure
Dr. Adriaenssens received educational grants from Abbott Vascular. Dr. Aguirre received research grants from Philips Healthcare, Inc. and Amgen, Inc. Dr. Akasaka received research grants from Abbott Vascular Japan, Nipro Inc., and Terumo Inc. and is a medical advisor for Terumo Inc. Dr. Ali: institutional grants from Abbott vascular, and cardiovascular system Inc. to Columbia University and Cardiovascular Research foundation; honoraria from Amgen, Astra Zeneca, Boston scientific; equity from Shockwave. Dr. Amabile received proctoring and consulting fees for Abbott Vascular & Boston Scientific; consulting fees for Shockwave Medical; institutional research grants from Abbott Vascular. Dr. Arbustini received grants from the Ministry of Health to the National IRCCS Cardiology Network (RCR-2019-23669116-001 and RCR-2020-23670065) and from FRRB grant CP_14/2018, INTESTRAT-CAD, Lombardia Region, Italy. Dr. Bouma has OCT patents, assigned to Massachusetts General Hospital and licensed to Terumo Corporation. Dr. Crea received personal fees from Amgen, personal fees from Astra Zeneca, personal fees from Servier, personal fees from BMS, other from GlyCardial Diagnostics, outside the submitted work. Dr. Dauerman is a consultant to Baim Clinical Research Institute, Cardiovascular Research Foundation, Medtronic and Boston Scientific; Dr. Dauerman has research grants from Medtronic and Boston Scientific. Dr. Di Mario received research grants (to the institution) from AMGEN, Behring, Boston Scientific, Chiesi, Daiichi-Sankyo, Edwards, Medtronic, Shockwave Volcano-Philips and speakers’ fees from Abbott and Shockwave. Dr. Finn and Dr. Virmani received institutional research support from NIH (HL141425), Leducq Foundation Grant; 480 Biomedical; 4C Medical; 4Tech; Abbott; Accumedical; Amgen; Biosensors; Boston Scientific; Cardiac Implants; Celonova; Claret Medical; Concept Medical; Cook; CSI; DuNing, Inc; Edwards LifeSciences; Emboline; Endotronix; Envision Scientific; Lutonix/Bard; Gateway; Lifetech; Limflo; MedAlliance; Medtronic; Mercator; Merill; Microport Medical; Microvention; Mitraalign; Mitra assist; NAMSA; Nanova; Neovasc; NIPRO; Novogate; Occulotech; OrbusNeich Medical; Phenox; Profusa; Protembis; Qool; Recor; Senseonics; Shockwave; Sinomed; Spectranetics; Surmodics; Symic; Vesper; W.L. Gore; Xeltis. A.V.F. has received honoraria from Abbott Vascular; Biosensors; Boston Scientific; Celonova; Cook Medical; CSI; Lutonix Bard; Sinomed; Terumo Corporation; and is a consultant to Amgen; Abbott Vascular; Boston Scientific; Celonova; Cook Medical; Lutonix Bard; Sinomed. R.V. has received honoraria from Abbott Vascular; Biosensors; Boston Scientific; Celonova; Cook Medical; Cordis; CSI; Lutonix Bard; Medtronic; OrbusNeich Medical; CeloNova; SINO Medical Technology; ReCore; Terumo Corporation; W. L. Gore; Spectranetics; and is a consultant Abbott Vascular; Boston Scientific; Celonova; Cook Medical; Cordis; CSI; Edwards Lifescience; Lutonix Bard; Medtronic; OrbusNeich Medical; ReCore; Sinomededical Technology; Spectranetics; Surmodics; Terumo Corporation; W. L. Gore; Xeltis. Dr. Fujimoto has financial interests in Optovue, receives royalties from intellectual property owned by MIT and licensed to Optovue and receives research support from Topcon and the National Institutes of Health. Dr. Garcia-Garcia recieved institutional grant support: Biotronik, Boston Scientific, Medtronic, Abbott, Neovasc, Shockwave, Philips, and CorFlow. Dr. Gerbaud is a consultant for Terumo and Abbott Vascular. Dr. Gonzalo received speaker and consultant fees from Abbott, speaker fees from Boston Scientific. Dr. Gori received speakeŕs honoraria and research support from Abbot vascular. Dr. Guagliumi received consultant fees from Abbott Vascular and Infraredx; research grant from Abbott Vascular, Infraredx, and Amgen. Dr. Hibi has received remuneration for lectures from Terumo, Abbott Vascular, and Boston Scientific Japan. Dr. Holm received speaker fees from Terumo, research grants and speaker fees from Abbott and Reva Medical, research grants from Boston Scientific, Biosensors, Bbraun, and Medis Medical Imaging. Dr. Jang received educational grants from Abbott Vascular and consulting fees from Svelte and Mitobridge. Dr. Jung-Sun Kim received proctoring fees from Abbott Vascular. Dr. Libby is an unpaid consultant to, or involved in clinical trials for Amgen, AstraZeneca, Baim Institute, Beren Therapeutics, Esperion Therapeutics, Genentech, Kancera, Kowa Pharmaceuticals, Medimmune, Merck, Norvo Nordisk, Novartis, Pfizer, Sanofi-Regeneron. Dr. Libby is a member of scientific advisory board for Amgen, Caristo, Cartesian, Corvidia Therapeutics, CSL Behring, DalCor Pharmaceuticals, Dewpoint, Kowa Pharmaceuticals, Olatec Therapeutics, Medimmune, Novartis, PlaqueTec, and XBiotech, Inc. Dr. Kume received personal fees from Abbott Japan Co., Ltd. Dr. Libby’s laboratory has received research funding in the last 2 years from Novartis. Dr. Libby is on the Board of Directors of XBiotech, Inc. Dr. Libby has a financial interest in Xbiotech, a company developing therapeutic human antibodies. Dr. Libby’s interests were reviewed and are managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict-of-interest policies. Dr. Libby’s laboratory has received research funding in the last 2 years from Novartis. Dr. Libby is on the Board of Directors of XBiotech, Inc. Dr. Libby has a financial interest in Xbiotech, a company developing therapeutic human antibodies. Dr. Libby’s interests were reviewed and are managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict-of-interest policies. Dr. Johnson received consultancy and speaker fees from Abbott Vascular & Terumo. Dr. Joner reports personal fees from Biotronik, personal fees from Orbus Neich, grants and personal fees from Boston Scientific, grants and personal fees from Edwards, personal fees from Recor, personal fees from Astra Zeneca, grants from Amgen, personal fees from Abbott, personal fees from Shockwave, grants from Infraredx, grants from Cardiac Dimensions outside the submitted work. Dr. Lerman received consultant fees from Volcano and Philips. Dr. Michalis received departmental grants from Abbott. Dr. Minami received an honorarium and consulting fee from Abbott. Dr. Mintz received honoraria from Boston Scientific, Philips/Volcano, Medtronic, Abiomed. Equity in SpectraWave. Dr. Nef received speaker honoraria and research grant from Abbott Vascular. Dr. Räber received grants to the institution by Abbott, Biotronik, BostonScientific, Heartflow, Sanofi, Regeneron and speaker/consultation fees by Abbott, Amgen, AstraZeneca, Canon, Occlutec, Sanofi, Vifor. Dr. Regar is a member of the medical advisory board for Zed Medical, Inc. and a clinical advisor for Kaminari Medical BV. Dr. Reynolds received donations for research from Abbott vascular, Siemens, and BioTelemetry. Dr. Shinke received research grant from Abbott Medical Japan. Dr. Souteyrand received consulting for Abbott medical, Terumo, Boston scientific, Medtronic, Schockwave. Dr. Stone received speaker honoraria from Terumo, Cook, Infraredx; consultant to Valfix, TherOx, Robocath, HeartFlow, Ablative Solutions, Vectorious, Miracor, Neovasc, Abiomed, Ancora, Elucid Bio, Occlutech, CorFlow, Reva, MAIA Pharmaceuticals, Vascular Dynamics, Shockwave, V-Wave, Cardiomech, Gore; equity/options from Ancora, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, Valfix, MedFocus family of funds. Dr. Tearney receives sponsored research funding from Canon, CN USA Biotech Holdings, VivoLight and AstraZeneca and catheter materials from Terumo Corporation. Dr. Tearney has a financial/fiduciary interest in SpectraWave, a company developing an OCT-NIRS intracoronary imaging system and catheter. His financial/fiduciary interest was reviewed and is managed by the Massachusetts General Hospital and Mass General Brigham HealthCare in accordance with their conflict of interest policies. Dr. Tearney (Terumo, Canon, Spectrawave) has the right to receive royalties from licensing arrangements. Dr. Toutouzas received research grants from Medtronic, and I am proctor for Medtronic and Abbott. Dr. Uemura received educational grants from Abbott Vascular Japan. Dr. Vergallo received speaker fees from Abbott. Dr. Weisz is a member of medical advisory board for Filterlex, Intratech, Microbot, and Trisol and received equity from Filterlex, Intratech, and Microbot and consulting fees from Filterlex, Intratech, Microbot, Magenta, and Cuspa. Dr. William: institutional research grant and honoraria from MicroPort (steering Committee TARGET AC trial; co-founder Argonauts, an innovation facilitator; medical advisor Rede Optimus Research and Corrib Core Laboratory, NUI Galway. Dr. Yan received research grant and speaker honorarium from Abbott Vascular. Dr. Yonetsu received endowment from Abbott Vascular Japan, Boston Scientific Japan, WIN International, Japan Lifeline, and Takeyama KK. Dr. Yu received research grants from the National Key R&D Program of China (2016YFC1301103) and the National Natural Science Foundation of China (81827806). The other writing committee members and coauthors declare that there is no conflict of interest.
Figures



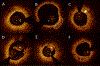
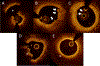


References
-
- Brezinski ME, Tearney GJ, Bouma BE, Izatt JA, Hee MR, Swanson EA, Southern JF, Fujimoto JG. Optical coherence tomography for optical biopsy. Properties and demonstration of vascular pathology. Circulation 1996;93(6):1206–13. - PubMed
-
- Jang IK, Bouma BE, Kang DH, Park SJ, Park SW, Seung KB, Choi KB, Shishkov M, Schlendorf K, Pomerantsev E, Houser SL, Aretz HT, Tearney GJ. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol 2002;39(4):604–9. - PubMed
-
- Yabushita H, Bouma BE, Houser SL, Aretz HT, Jang I-K, Schlendorf KH, Kauffman CR, Shishkov M, Kang D-H, Halpern EF, Tearney GJ. Characterization of Human Atherosclerosis by Optical Coherence Tomography. Circulation 2002;106(13):1640–1645. - PubMed
-
- Raber L, Mintz GS, Koskinas KC, Johnson TW, Holm NR, Onuma Y, Radu MD, Joner M, Yu B, Jia H, Meneveau N, de la Torre Hernandez JM, Escaned J, Hill J, Prati F, Colombo A, di Mario C, Regar E, Capodanno D, Wijns W, Byrne RA, Guagliumi G, Group ESCSD. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J 2018;39(35):3281–3300. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

