Longitudinal gut virome analysis identifies specific viral signatures that precede necrotizing enterocolitis onset in preterm infants
- PMID: 35449461
- PMCID: PMC9064801
- DOI: 10.1038/s41564-022-01096-x
Longitudinal gut virome analysis identifies specific viral signatures that precede necrotizing enterocolitis onset in preterm infants
Abstract
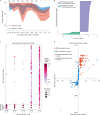
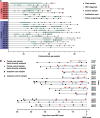
Necrotizing enterocolitis (NEC) is a serious consequence of preterm birth and is often associated with gut bacterial microbiome alterations. However, little is known about the development of the gut virome in preterm infants, or its role in NEC. Here, using metagenomic sequencing, we characterized the DNA gut virome of 9 preterm infants who developed NEC and 14 gestational age-matched preterm infants who did not. Infants were sampled longitudinally before NEC onset over the first 11 weeks of life. We observed substantial interindividual variation in the gut virome between unrelated preterm infants, while intraindividual variation over time was significantly less. We identified viral and bacterial signatures in the gut that preceded NEC onset. Specifically, we observed a convergence towards reduced viral beta diversity over the 10 d before NEC onset, which was driven by specific viral signatures and accompanied by specific viral-bacterial interactions. Our results indicate that bacterial and viral perturbations precede the sudden onset of NEC. These findings suggest that early life virome signatures in preterm infants may be implicated in NEC.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures