Alveolar Hemorrhage Caused by the Combination of Immune Checkpoint Inhibitors (ICIs) and Angiogenesis Inhibitors: The Underlying Long-Term Vascular Endothelial Growth Factor (VEGF) Inhibition
- PMID: 35449623
- PMCID: PMC9012575
- DOI: 10.7759/cureus.23272
Alveolar Hemorrhage Caused by the Combination of Immune Checkpoint Inhibitors (ICIs) and Angiogenesis Inhibitors: The Underlying Long-Term Vascular Endothelial Growth Factor (VEGF) Inhibition
Abstract
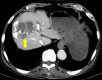
The combination of immune checkpoint inhibitors (ICIs) and other anticancer agents is the standard of care for various cancers. Bevacizumab, an anti-angiogenesis inhibitor, causes serious adverse events such as pulmonary hemorrhage (PH). Here, we present a case of drug-induced diffuse alveolar hemorrhage (DAH), an adverse event, in a patient with hepatocellular carcinoma who was treated with a combination of ICIs and anti-angiogenesis inhibitors after long-term use of lenvatinib, which inhibits vascular endothelial growth factor (VEGF). An 85-year-old man with hepatocellular carcinoma initially received lenvatinib, a multi-kinase inhibitor, but the drug was later switched to bevacizumab-atezolizumab combination therapy owing to disease progression. After five cycles, he developed dyspnea and diffuse ground-glass opacities, which improved with discontinuation of the combination therapy and initiation of steroid pulse therapy. Our case findings indicate that both ICIs and anti-angiogenesis inhibitors cause drug-induced DAH, and their combination may increase the severity of DAH. Moreover, long-term VEGF inhibition may induce the development of DAH. Clinicians need to be aware that long-term VEGF inhibition may be associated with DAH and should consider the risk management of such adverse events while using this combination therapy.
Keywords: adverse events; anti-angiogenesis inhibitor; combination therapy; diffuse alveolar hemorrhage; immune checkpoint inhibitor; long-term vegf inhibition.
Copyright © 2022, Shijubou et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Updated analysis of KEYNOTE- 024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. Reck M, Rodríguez-Abreu D, Robinson AG, et al. J Clin Oncol. 2019;37:537–546. - PubMed
-
- Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Lancet Oncol. 2018;19:1480–1492. - PubMed
-
- Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Galluzzi L, Humeau J, Buqué A, Zitvogel L, Kroemer G. Nat Rev Clin Oncol. 2020;17:725–741. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
