Evaluation of the Pro-, Anti-Inflammatory, and Anabolic Effects of Autologous Platelet-Rich Gel Supernatants in an in vitro Coculture System of Canine Osteoarthritis
- PMID: 35449726
- PMCID: PMC9017510
- DOI: 10.1155/2022/3377680
Evaluation of the Pro-, Anti-Inflammatory, and Anabolic Effects of Autologous Platelet-Rich Gel Supernatants in an in vitro Coculture System of Canine Osteoarthritis
Abstract
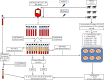
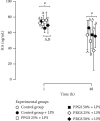
There are scarce in vitro studies indicating the basic mechanisms of why platelet-rich plasma (PRP) is useful in the clinical management of dogs with naturally occurring OA. Methods. Cartilage and synovial membrane explants from six dogs were challenged with lipopolysaccharide (LPS) and cultured for 48 h with platelet-poor gel supernatant (PPGS) and platelet-rich gel supernatant (PRGS) at concentrations of 25 and 50%, respectively. The tissue explants challenged with LPS were cocultured over 48 h and culture media were sampled at 1 and 48 h for the determination of IL-1β, IL-10, hyaluronan, TGF-β1, and PDGF-BB by ELISA. Results. IL-1β concentrations were significantly higher in tissue explant groups cultured for 48 h with PRGS at 50% and with PPGS at 25% when compared to the remaining experimental groups at any time. IL-10 and HA presented similar concentrations in all evaluated groups at any time. TGF-β1 and PDGF-BB presented higher concentrations in the culture media of tissue explants cultured with PPGS and PRGS at 50%, which diminished with time. Conclusions. Both PPGS and PRGS at both concentrations showed a limited biological effect on cartilage and synovial membrane explants in coculture with LPS. Even PPGS at 25% and PRGS at 50% exhibited proinflammatory effects on these tissues at 48 h.
Copyright © 2022 Miller Gallego et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures




References
-
- Cimino Brown D. What can we learn from osteoarthritis pain in companion animals? Clinical & Experimental Rheumatology . 2017;35(5):53–58. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

