The Combination of Zerumbone with 5-Fluorouracil for Sensitizing Colorectal Cancer-Associated Fibroblasts to Treatment
- PMID: 35449812
- PMCID: PMC9017496
- DOI: 10.1155/2022/9369328
The Combination of Zerumbone with 5-Fluorouracil for Sensitizing Colorectal Cancer-Associated Fibroblasts to Treatment
Abstract
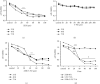
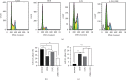
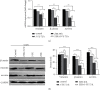
The present study aimed to evaluate the synergic effects of combination therapy on 5-fluorouracil (5-FU) resistance-cancer-associated fibroblasts (CAFs) to treatment. Chemotherapy resistance is an important challenge in colorectal cancer (CRC) eradication attention to the tumor microenvironment (TME) is very important. CAFs in the TME play an essential role in cancer chemoresistance and relapse. Additionally, many patients with advanced CRC show resistance to 5-FU therapy. Anti-tumorigenic activities of ZER, a chemopreventive compound derived from the rhizomes of the wild ginger, have been demonstrated. Synergistic and potentiating effects of combination therapy, using herbal and chemical drugs, can improve patients' response. At the first, CAFs were isolated from a CRC patient and sorted by fluorescent-activated cell sorting (FACS), then, confirmed by flow cytometry, and immunocytochemistry (ICC). The effect of 5-FU and ZER on the cell viability was investigated by MTT assay in a dose and time-dependent manner, after that, the expression of vimentin, β-catenin, and survivin was quantified. Apoptosis, cell cycle, and invasion were analyzed by flow cytometry and scratch test, respectively. ZER could significantly sensitize CAFs cells to 5-FU. A combination of 5-FU + ZER revealed a marked decrease in the marker of interest in both mRNA and protein levels compared to control groups, including 5-FU, ZER treated, and untreated cells. Functional evaluation of cells in different groups presented significant suppression in migration of CAFs and an apparent increase in cell arrest and apoptosis by 5-FU + ZER treatment.
Copyright © 2022 Sima Nobari et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
LinkOut - more resources
Full Text Sources

