Detection of Serotonin, Melatonin, and Their Metabolites in Honey
- PMID: 35449872
- PMCID: PMC9017714
- DOI: 10.1021/acsfoodscitech.1c00119
Detection of Serotonin, Melatonin, and Their Metabolites in Honey
Abstract
Melatonin and serotonin, products of tryptophan metabolism, are endogenous neurotransmitters and hormones. We have identified and quantified these metabolites in natural honey from Australia, USA, and Poland using a Xevo G2 XS qTof LC-MS. To help ensure correct product identification, some samples were prepurified by RP-HPLC based on the retention times of standards, prior to LC-MS. The concentrations of the metabolites of interest depended on the source of the honey. For Australian honey, levels for melatonin and 2-hydroxymelatonin were 0.91 and 0.68 ng/g, respectively. Melatonin was detected in one brand of US commercial honey at 0.48 ng/g, while a second brand contained serotonin at 88.2 ng/g. In Polish natural honey, 20.6 ng/g of serotonin and 40.8 ng/g of N-acetylserotonin (NAS) were detected, while in Polish commercial honey 25.9 ng/g of serotonin and 7.30 ng/g of NAS were present. We suggest that addictive and health-related properties of honey may be in part dependent on the presence of serotonin, melatonin, and their metabolites, and that these compounds may play a role in the colony activities of bees.
Keywords: 2-hydroxymelatonin; N-acetylserotonin; bees; honey; melatonin; serotonin.
Figures


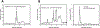
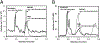
References
-
- Tan DX; Hardeland R; Manchester LC; Korkmaz A; Ma S; Rosales-Corral S; Reiter RJ Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot 2012, 63 (2), 577–97. - PubMed
-
- Tan DX; Manchester LC; Liu X; Rosales-Corral SA; Acuna-Castroviejo D; Reiter RJ Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin’s primary function and evolution in eukaryotes. J. Pineal Res 2013, 54 (2), 127–38. - PubMed
-
- Back K; Tan DX; Reiter RJ Melatonin biosynthesis in plants: multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res 2016, 61 (4), 426–437. - PubMed
-
- Cipolla-Neto J; Amaral F. G. d. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev 2018, 39 (6), 990–1028. - PubMed
-
- Tan D; Reiter RJ Mitochondria: the birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2019, 2 (1), 44–66.
Grants and funding
LinkOut - more resources
Full Text Sources
