Removal of Uranium-238, Thorium-232, and Potassium-40 from Wastewater via Adsorption on Multiwalled Carbon Nanotubes
- PMID: 35449914
- PMCID: PMC9016888
- DOI: 10.1021/acsomega.2c00819
Removal of Uranium-238, Thorium-232, and Potassium-40 from Wastewater via Adsorption on Multiwalled Carbon Nanotubes
Abstract
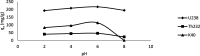
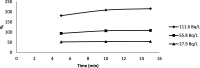
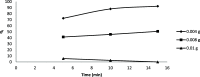
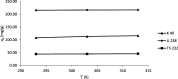
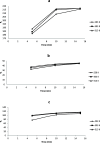
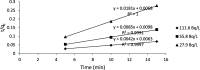
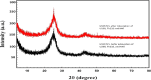
The optimum conditions for the removal of uranium-238, thorium-232, and potassium-40 from wastewater and the discharge of nuclear facilities using multiwalled carbon nanotubes (CNTs) are described. The adsorption mechanism is mainly attributed to chemical interactions between the metal ions and surface functional groups of the CNTs. Batch adsorption experiments are carried out in order to study the effect of different parameters such as pH, contact time, initial metal ion concentration, adsorbent dose, and temperatures. Maximum metal removal (>98%) from solutions containing 20-120 Bq/L metal ions is achieved using a contact time of 15 min, a pH of 6.0, and 10 mg/L CNTs. The effect of temperature on the kinetics and equilibrium of adsorption on CNT particles is examined. Consistent with an exothermic reaction, an increase in the temperature resulted in an increase in the adsorption rate. Langmuir, Freundlich, and Dubinin-Radushkevich isotherms are applied to the data obtained at various temperatures. The Langmuir adsorption model is the best for data interpretations. The kinetics of adsorption reveals a pseudo-second-order mechanism. Thermodynamic parameters at 293 K (ΔG°, ΔH°, and ΔS°) for U-238, Th-232, and K-40 are -14590.7 kJ/mol, -6.66 kJ/mol, and 26.47 J/(mol K), -96,96.5 kJ/mol, -2.48 kJ/mol, and 14.17 J/(mol K), and -3922.09 kJ/mol, -1.32 kJ/mol, and 6.12 J/(mol K), respectively.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures























References
-
- Ojovan M. I.; Lee W. E.; Kalmykov S. N.. An Introduction to Nuclear Waste Immobilisation, 3rd ed.; Elsevier, 2019.
-
- Al-Khawlany A.; Khan A.; Pathan J. Review on studies in natural background radiation. Radiat. Protect. Environ. 2018, 41, 215–222. 10.4103/rpe.rpe_55_18. - DOI
-
- Oberender A.; Juel Lyng R.; Lyngfelt Molander L.; Jensen C.; Schouenborg B.; Theorin M.; Wærner E. R.; Røgeberg M.; Punkkinen H.; Wahlström M.. Survey of the Emergence and Use of Naturally Occurring Materials; Nordic Council of Ministers, 2021.
-
- Labrincha J.; Puertas F.; Schroeyers W.; Kovler K.; Pontikes Y.; Nuccetelli C.; Krivenko P.; Kovalchuk O.; Petropavlovsky O.; Komljenovic M.; Fidanchevski E.. From NORM by-products to building materials. Naturally Occurring Radioactive Materials in Construction; Woodhead Publishing, 2017; pp 183–252.
-
- Nieder R.; Benbi D. K.; Reichl F. X.. Health Risks Associated with Radionuclides in Soil Materials. Soil Components and Human Health; Springer: Dordrecht, 2018; pp 451–501.
LinkOut - more resources
Full Text Sources

