Liraglutide Attenuates Hepatic Ischemia-Reperfusion Injury by Modulating Macrophage Polarization
- PMID: 35450076
- PMCID: PMC9016191
- DOI: 10.3389/fimmu.2022.869050
Liraglutide Attenuates Hepatic Ischemia-Reperfusion Injury by Modulating Macrophage Polarization
Abstract
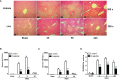
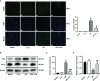
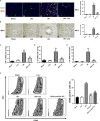
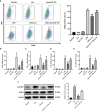
Ischemia-reperfusion injury (IRI) is a common complication associated with liver surgery, and macrophages play an important role in hepatic IRI. Liraglutide, a glucagon-like peptide-1 (GLP-1) analog primarily used to treat type 2 diabetes and obesity, regulates intracellular calcium homeostasis and protects the cardiomyocytes from injury; however, its role in hepatic IRI is not yet fully understood. This study aimed to investigate whether liraglutide can protect the liver from IRI and determine the possible underlying mechanisms. Our results showed that liraglutide pretreatment significantly alleviated the liver damage caused by ischemia-reperfusion (I/R), as evidenced by H&E staining, serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels, and TUNEL staining. Furthermore, the levels of inflammatory cytokines elicited by I/R were distinctly suppressed by liraglutide pretreatment, accompanied by significant reduction in TNF-α, IL-1β, and IL-6 levels. Furthermore, pretreatment with liraglutide markedly inhibited macrophage type I (M1) polarization during hepatic IRI, as revealed by the significant reduction in CD68+ levels in Kupffer cells (KCs) detected via flow cytometry. However, the protective effects of liraglutide on hepatic IRI were partly diminished in GLP-1 receptor-knockout (GLP-1R-/-) mice. Furthermore, in an in vitro study, we assessed the role of liraglutide in macrophage polarization by examining the expression profiles of M1 in bone marrow-derived macrophages (BMDMs) from GLP-1R-/- and C57BL/6J mice. Consistent with the results of the in vivo study, liraglutide treatment attenuated the LPS-induced M1 polarization and reduced the expression of M1 markers. However, the inhibitory effect of liraglutide on LPS-induced M1 polarization was largely abolished in BMDMs from GLP-1R-/- mice. Collectively, our study indicates that liraglutide can ameliorate hepatic IRI by inhibiting macrophage polarization towards an inflammatory phenotype via GLP-1R. Its protective effect against liver IRI suggests that liraglutide may serve as a potential drug for the clinical treatment of liver IRI.
Keywords: acute liver injury; glucagon-like peptide-1 receptor; ischemia-reperfusion; liraglutide; macrophage polarization.
Copyright © 2022 Li, Wang, Xu, Che, Hu, Cao, Xie, Qiu, Shi, Liu, Dai and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

