Implementation of a Rural Community Diagnostic Testing Strategy for SARS-CoV-2 in Upstate South Carolina
- PMID: 35450120
- PMCID: PMC9016164
- DOI: 10.3389/fpubh.2022.858421
Implementation of a Rural Community Diagnostic Testing Strategy for SARS-CoV-2 in Upstate South Carolina
Abstract
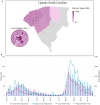
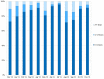
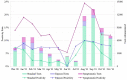
By developing a partnership amongst a public university lab, local city government officials and community healthcare providers, we established a drive-through COVID-19 testing site aiming to improve access to SARS-CoV-2 testing in rural Upstate South Carolina. We collected information on symptoms and known exposures of individuals seeking testing to determine the number of pre- or asymptomatic individuals. We completed 71,102 SARS-CoV-2 tests in the community between December 2020-December 2021 and reported 91.49% of results within 24 h. We successfully identified 5,244 positive tests; 73.36% of these tests originated from individuals who did not report symptoms. Finally, we identified high transmission levels during two major surges and compared test positivity rates of the local and regional communities. Importantly, the local community had significantly lower test positivity rates than the regional community throughout 2021 (p < 0.001). While both communities reached peak case load and test positivity near the same time, the local community returned to moderate transmission as indicated by positivity 4 weeks before the regional community. Our university lab facilitated easy testing with fast turnaround times, which encouraged voluntary testing and helped identify a large number of non-symptomatic cases. Finding the balance of simplicity, accessibility, and community trust was vital to the success of our widespread community testing program for SARS-CoV-2.
Keywords: COVID-19; SARS-CoV-2; community health; rural; saliva testing; surveillance.
Copyright © 2022 Plumb, Ham, Napolitano, King, Swann, Kalbaugh, Rennert and Dean.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- South Carolina Department of Health and Environmental Control . COVID-19 Data and Dashboards (2021). Available online at: https://scdhec.gov/covid19/covid-19-data (accessed December 19, 2021).
-
- Chu AW, Chan W, Ip JD, Yip CC, Chan JF, Yuen K, et al. Evaluation of simple nucleic acid extraction methods for the detection of SARS-CoV-2 in nasopharyngeal and saliva specimens during global shortage of extraction kits. J Clin Virol. (2020) 129:104519. 10.1016/j.jcv.2020.104519 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

