Three reasons why expanded use of natural enemy solutions may offer sustainable control of human infections
- PMID: 35450207
- PMCID: PMC9017516
- DOI: 10.1002/pan3.10264
Three reasons why expanded use of natural enemy solutions may offer sustainable control of human infections
Abstract
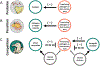
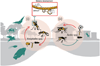
1. Many infectious pathogens spend a significant portion of their life cycles in the environment or in animal hosts, where ecological interactions with natural enemies may influence pathogen transmission to people. Yet, our understanding of natural enemy opportunities for human disease control is lacking, despite widespread uptake and success of natural enemy solutions for pest and parasite management in agriculture. 2. Here we explore three reasons why conserving, restoring, or augmenting specific natural enemies in the environment could offer a promising complement to conventional clinical strategies to fight environmentally mediated pathogens and parasites. (1) Natural enemies of human infections abound in nature, largely understudied and undiscovered. (2) Natural enemy solutions could provide ecological options for infectious disease control where conventional interventions are lacking. And, (3) Many natural enemy solutions could provide important co-benefits for conservation and human well-being. 3. We illustrate these three arguments with a broad set of examples whereby natural enemies of human infections have been used or proposed to curb human disease burden, with some clear successes. However, the evidence base for most proposed solutions is sparse, and many opportunities likely remain undiscovered, highlighting opportunities for future research.
Keywords: biological control; disease control; disease ecology; infectious disease; natural enemies; sustainability; sustainable development.
Conflict of interest statement
Conflict of Interest: The authors declare no conflicts of interest.
Figures

Similar articles
-
Pest control of aphids depends on landscape complexity and natural enemy interactions.PeerJ. 2015 Jul 16;3:e1095. doi: 10.7717/peerj.1095. eCollection 2015. PeerJ. 2015. PMID: 26734497 Free PMC article.
-
The distribution of covert microbial natural enemies of a globally invasive crop pest, fall armyworm, in Africa: Enemy release and spillover events.J Anim Ecol. 2022 Sep;91(9):1826-1841. doi: 10.1111/1365-2656.13760. Epub 2022 Jun 22. J Anim Ecol. 2022. PMID: 35678697 Free PMC article.
-
Assessing the impact of arthropod natural enemies on crop pests at the field scale.Insect Sci. 2015 Feb;22(1):20-34. doi: 10.1111/1744-7917.12174. Epub 2014 Dec 2. Insect Sci. 2015. PMID: 25219624 Review.
-
Defence against multiple enemies.J Evol Biol. 2003 Nov;16(6):1319-27. doi: 10.1046/j.1420-9101.2003.00585.x. J Evol Biol. 2003. PMID: 14640423
-
Improving Natural Enemy Selection in Biological Control through Greater Attention to Chemical Ecology and Host-Associated Differentiation of Target Arthropod Pests.Insects. 2022 Feb 2;13(2):160. doi: 10.3390/insects13020160. Insects. 2022. PMID: 35206733 Free PMC article. Review.
Cited by
-
Green Nano-Biotechnology: A New Sustainable Paradigm to Control Dengue Infection.Bioinorg Chem Appl. 2022 Aug 8;2022:3994340. doi: 10.1155/2022/3994340. eCollection 2022. Bioinorg Chem Appl. 2022. PMID: 35979184 Free PMC article. Review.
-
Temperature affects predation of schistosome-competent snails by a novel invader, the marbled crayfish Procambarus virginalis.PLoS One. 2023 Sep 13;18(9):e0290615. doi: 10.1371/journal.pone.0290615. eCollection 2023. PLoS One. 2023. PMID: 37703262 Free PMC article.
References
-
- Amora SSA, Bevilaqua CML, Feijo FMC, Silva MA, Pereira R, Silva SC, Alves ND, Freire FAM, & Oliveira DM (2009). Evaluation of the fungus Beauveria bassiana (Deuteromycotina: Hyphomycetes), a potential biological control agent of Lutzomyia longipalpis (Diptera, Psychodidae). Biological Control, 50(3), 329–335. 10.1016/j.biocontrol.2009.05.004 - DOI
-
- Barratt BIP, Moran VC, Bigler F, & van Lenteren JC (2018). The status of biological control and recommendations for improving uptake for the future. BioControl, 63(1), 155–167. 10.1007/s10526-017-9831-y - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
