Immunogenicity Associated with Aesthetic Botulinumtoxin A: A Survey of Asia-Pacific Physicians' Experiences and Recommendations
- PMID: 35450268
- PMCID: PMC9015201
- DOI: 10.1097/GOX.0000000000004217
Immunogenicity Associated with Aesthetic Botulinumtoxin A: A Survey of Asia-Pacific Physicians' Experiences and Recommendations
Abstract
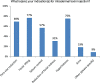
Background: Most botulinum toxin A (BoNT/A) products contain unnecessary bacterial components that increase the risk of developing neutralizing antibodies (nAbs). Reports of secondary nonresponse and treatment failures (STF) due to nAbs have accompanied a surge in new BoNT/A products.
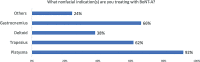
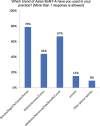
Methods: To formulate recommendations on managing toxin resistance, we reviewed the evidence on BoNT/A-associated immunogenicity and evaluated Asian physicians' current BoNT/A practices, knowledge, and real-world experiences, as provided by survey outcomes conducted with 128 Asian experts (regular botulinum toxin injectors).
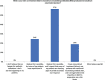
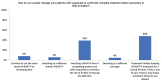
Results: Most doctors believe STF occurs, some patients exhibit partial symptoms, and impurities (eg, complexing proteins) in BoNT/A preparations risk STF. Bioassays that distinguish non-nAbs from nAbs that hinder toxin function remain unavailable to most doctors, though most would perform testing if given the option. Doctors in the Asia-Pacific region have differing strategies for managing STF, depending on the availability of alternatives or tests. They recommended switching to a highly-purified formulation free of complexing proteins and other impurities to lower the risk of immunogenicity, or offering treatment holidays of 2 -2.5 years. They suggested restarting treatment with the same highly purified formulation, especially for repeated treatments, large-dose injections, and younger patients who will accumulate higher lifetime doses, so as to minimize immunogenic risks and preserve long-term treatment outcomes. Importantly, doctors should always initiate patients on pure formulations rather than switching to these only after resistance develops.
Conclusion: Choosing highly purified BoNT/A products at treatment initiation enhances long-term efficacy and patient satisfaction while minimizing the risk of immune activation and nAb formation.
Copyright © 2022 The Authors. Published by Wolters Kluwer Health, Inc. on behalf of The American Society of Plastic Surgeons.
Figures

References
-
- Kerscher M, Wanitphakdeedecha R, Trindade de Almeida A, et al. . IncobotulinumtoxinA: a highly purified and precisely manufactured botulinum neurotoxin type A. J Drugs Dermatol. 2019;18:52–57. - PubMed
-
- Jeong S. 4 new botulinum toxins to undergo trials in crowded market. Available at https://www.koreabiomed.com/news/articleView.html?idxno=7021. Published 2020. Accessed September 28, 2020.
-
- Lim J. Wrinkle treatment companies vie to fill hole left by Meditoxin. Available at http://www.koreaherald.com/view.php?ud=20200506000924. Published 2020. Accessed September 28, 2020.
LinkOut - more resources
Full Text Sources
