Induction of Filopodia During Cytomegalovirus Entry Into Human Iris Stromal Cells
- PMID: 35450284
- PMCID: PMC9018114
- DOI: 10.3389/fmicb.2022.834927
Induction of Filopodia During Cytomegalovirus Entry Into Human Iris Stromal Cells
Abstract
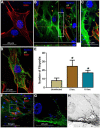
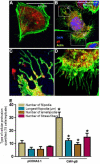
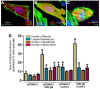
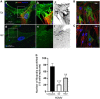
Many viruses exploit thin projections of filopodia for cell entry and cell-to-cell spread. Using primary cultures of human iris stromal (HIS) cells derived from human eye donors, we report a significant increase in filopodia formation during human cytomegalovirus (HCMV) infection. Using confocal microscopy, we observed a large number of virions being frequently associated along the filopodia prior to cell infection. Depolymerization of actin filaments resulted in a significant inhibition of HCMV entry into HIS cell. Our results further revealed that the transient expression of HCMV envelope glycoprotein B (gB) triggers the induction of the filopodial system. Since gB is known to bind the diverse chains of heparan sulfate (HS), a comparative study was performed to evaluate the gB-mediated filopodial induction in cells expressing either wild-type HS and/or 3-O sulfated HS (3-OS HS). We found that cells co-expressing HCMV gB together with the 3-O sulfotranseferase-3 (3-OST-3) enzyme had a much higher and robust filopodia induction compared to cells co-expressing gB with wild-type HS. The above results were further verified by pre-treating HIS cells with anti-3-OS HS (G2) peptide and/or heparinase-I before challenging with HCMV infection, which resulted in a significant loss in the filopodial counts as well as decreased viral infectivity. Taken together, our findings highlight that HCMV entry into HIS cells actively modulates the actin cytoskeleton via coordinated actions possibly between gB and the 3-OS HS receptor to influence viral infectivity.
Keywords: 3-O sulfated heparan sulfate; actin cytoskeletal network; heparan sulfate; virus cell to cell spread; virus entry.
Copyright © 2022 Chang, Majmudar, Tandon, Volin and Tiwari.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources

