Covalently grafting first-generation PAMAM dendrimers onto MXenes with self-adsorbed AuNPs for use as a functional nanoplatform for highly sensitive electrochemical biosensing of cTnT
- PMID: 35450327
- PMCID: PMC8967855
- DOI: 10.1038/s41378-022-00352-8
Covalently grafting first-generation PAMAM dendrimers onto MXenes with self-adsorbed AuNPs for use as a functional nanoplatform for highly sensitive electrochemical biosensing of cTnT
Abstract
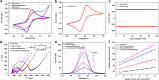
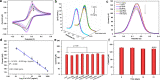
2D MXene-Ti3C2Tχ has demonstrated promising application prospects in various fields; however, it fails to function properly in biosensor setups due to restacking and anodic oxidation problems. To expand beyond these existing limitations, an effective strategy to for modifying the MXene by covalently grafting first-generation poly(amidoamine) dendrimers onto an MXene in situ (MXene@PAMAM) was reported herein. When used as a conjugated template, the MXene not only preserved the high conductivity but also conferred a specific 2D architecture and large specific surface areas for anchoring PAMAM. The PAMAM, an efficient spacer and stabilizer, simultaneously suppressed the substantial restacking and oxidation of the MXene, which endowed this hybrid with improved electrochemical performance compared to that of the bare MXene in terms of favorable conductivity and stability under anodic potential. Moreover, the massive amino terminals of PAMAM offer abundant active sites for adsorbing Au nanoparticles (AuNPs). The resulting 3D hierarchical nanoarchitecture, AuNPs/MXene@PAMAM, had advanced structural merits that led to its superior electrochemical performance in biosensing. As a proof of concept, this MXene@PAMAM-based nanobiosensing platform was applied to develop an immunosensor for detecting human cardiac troponin T (cTnT). A fast, sensitive, and highly selective response toward the target in the presence of a [Fe(CN)6]3-/4- redox marker was realized, ensuring a wide detection of 0.1-1000 ng/mL with an LOD of 0.069 ng/mL. The sensor's signal only decreased by 4.38% after 3 weeks, demonstrating that it exhibited satisfactory stability and better results than previously reported MXene-based biosensors. This work has potential applicability in the bioanalysis of cTnT and other biomarkers and paves a new path for fabricating high-performance MXenes for biomedical applications and electrochemical engineering.
Keywords: Biosensors; Electronic properties and materials.
© The Author(s) 2022.
Conflict of interest statement
Conflict of interestThe authors declare no competing interests.
Figures




Similar articles
-
Electrochemical biosensor based on PAMAM functionalized MXene nanoplatform for detection of folate receptor.Bioelectrochemistry. 2024 Apr;156:108627. doi: 10.1016/j.bioelechem.2023.108627. Epub 2023 Dec 16. Bioelectrochemistry. 2024. PMID: 38142545
-
Ti3C2 MXene mediated Prussian blue in situ hybridization and electrochemical signal amplification for the detection of exosomes.Talanta. 2021 Mar 1;224:121879. doi: 10.1016/j.talanta.2020.121879. Epub 2020 Nov 10. Talanta. 2021. PMID: 33379088
-
Ratiometric Antifouling Electrochemical Biosensors Based on Multifunctional Peptides and MXene Loaded with Au Nanoparticles and Methylene Blue.ACS Appl Mater Interfaces. 2021 May 5;13(17):20388-20396. doi: 10.1021/acsami.1c04933. Epub 2021 Apr 20. ACS Appl Mater Interfaces. 2021. PMID: 33878863
-
Electrochemical and optical biosensors based on multifunctional MXene nanoplatforms: Progress and prospects.Talanta. 2021 Dec 1;235:122726. doi: 10.1016/j.talanta.2021.122726. Epub 2021 Jul 21. Talanta. 2021. PMID: 34517594 Review.
-
Poly(amidoamine) (PAMAM): An emerging material for electrochemical bio(sensing) applications.Talanta. 2016 Feb 1;148:427-38. doi: 10.1016/j.talanta.2015.11.022. Epub 2015 Nov 10. Talanta. 2016. PMID: 26653469 Review.
Cited by
-
Green synthesized apigenin conjugated gold nanoparticles inhibit cholangiocarcinoma cell activity and endothelial cell angiogenesis in vitro.Heliyon. 2022 Dec 1;8(12):e12028. doi: 10.1016/j.heliyon.2022.e12028. eCollection 2022 Dec. Heliyon. 2022. PMID: 36506385 Free PMC article.
-
Recent Trends in Metal Nanoparticles Decorated 2D Materials for Electrochemical Biomarker Detection.Biosensors (Basel). 2023 Jan 5;13(1):91. doi: 10.3390/bios13010091. Biosensors (Basel). 2023. PMID: 36671926 Free PMC article. Review.
-
Surface-Functionalizing Strategies for Multiplexed Molecular Biosensing: Developments Powered by Advancements in Nanotechnologies.Nanomaterials (Basel). 2024 Dec 14;14(24):2014. doi: 10.3390/nano14242014. Nanomaterials (Basel). 2024. PMID: 39728549 Free PMC article. Review.
-
Towards hospital-on-chip supported by 2D MXenes-based 5th generation intelligent biosensors.Biosens Bioelectron. 2023 Jan 15;220:114847. doi: 10.1016/j.bios.2022.114847. Epub 2022 Oct 27. Biosens Bioelectron. 2023. PMID: 36335709 Free PMC article. Review.
-
Surface Modification of Biochar to Prepare Environmentally Friendly Electrochemical Biosensors for Detection of Cardiac Troponin T.ACS Omega. 2025 Jun 9;10(24):25842-25854. doi: 10.1021/acsomega.5c02113. eCollection 2025 Jun 24. ACS Omega. 2025. PMID: 40584307 Free PMC article.
References
-
- Thomas H, et al. Global Atlas of Cardiovascular Disease 2000–2016: the path to prevention and control. Glob. Heart. 2018;13:143–163. - PubMed
-
- Qian X, et al. Facile and clean synthesis of dihydroxylatopillar[5]arene-stabilized gold nanoparticles integrated Pd/MnO2 nanocomposites for robust and ultrasensitive detection of cardiac troponin I. Biosens. Bioelectron. 2019;130:214–224. - PubMed
-
- Ye J, et al. Dual-wavelength ratiometric electrochemiluminescence immunosensor for cardiac troponin I detection. Anal. Chem. 2018;91:1524–1531. - PubMed
-
- Hamm C. W. et al. Emergency room triage of patients with acute chest pain by means of rapid testing for cardiac troponin T or troponin I. N. Engl. J. Med. 337, 1648–1653 (1997). - PubMed
-
- Qureshi A, Gurbuz Y, Niazi JH. Biosensors for cardiac biomarkers detection: a review. Sensors Actuat. B Chem. 2012;171-172:62–76.
LinkOut - more resources
Full Text Sources
Research Materials
