Diminishing GSH-Adduct Formation of Tricyclic Diazepine-based Mutant IDH1 Inhibitors
- PMID: 35450359
- PMCID: PMC9014435
- DOI: 10.1021/acsmedchemlett.2c00089
Diminishing GSH-Adduct Formation of Tricyclic Diazepine-based Mutant IDH1 Inhibitors
Abstract
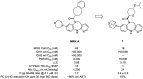
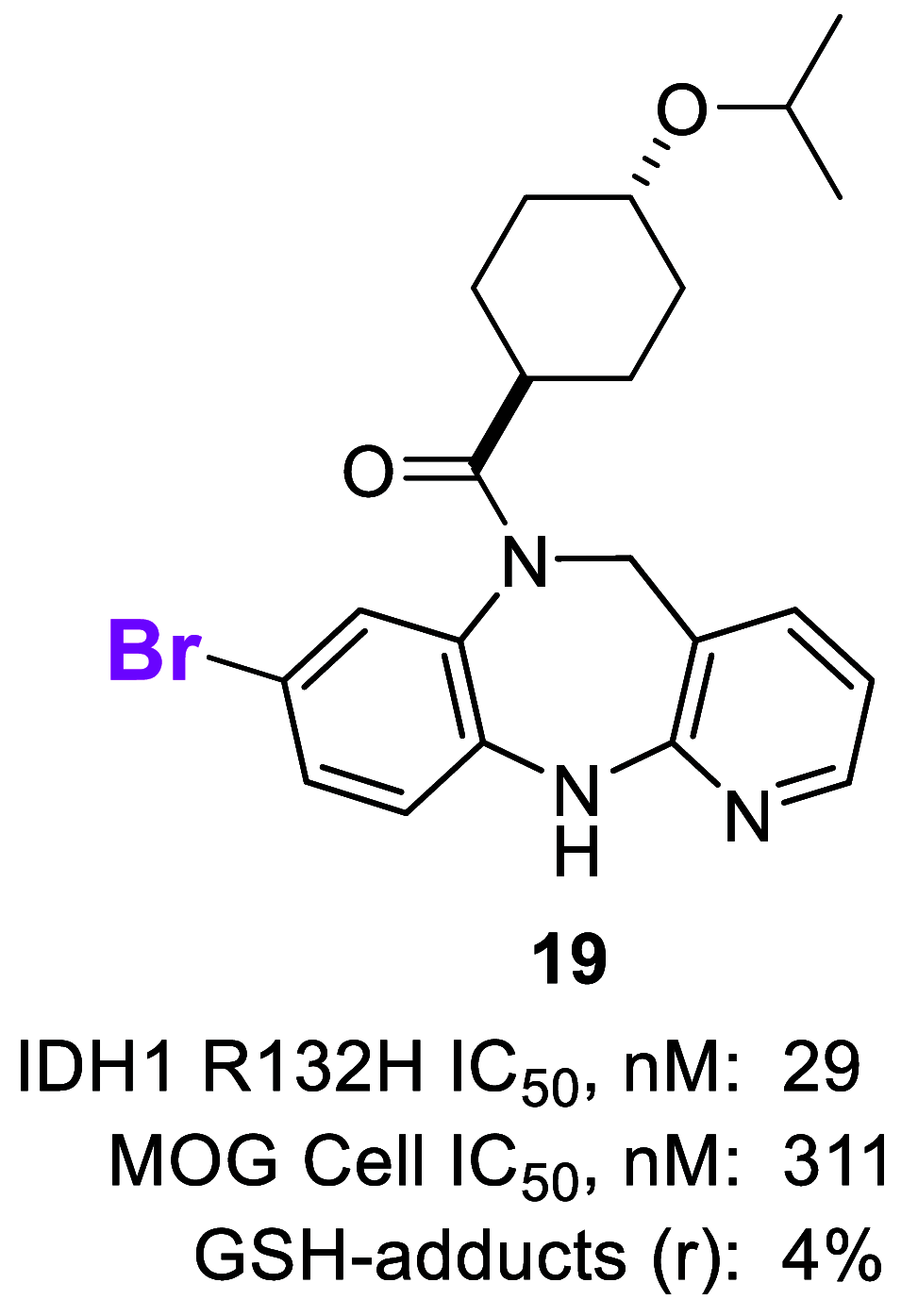
Mutant isocitrate dehydrogenase 1 (IDH1) has been identified as an attractive oncology target for which >70% of grade II and III gliomas and ∼10% of acute myeloid leukemia (AML) harbor somatic IDH1 mutations. These mutations confer a neomorphic gain of function, leading to the production of the oncometabolite (R)-2-hydroxyglutarate (2-HG). We identified and developed a potent, selective, and orally bioavailable brain-penetrant tricyclic diazepine scaffold that inhibits mutant IDH1. During the course of in vitro metabolism studies, GSH-adduct metabolites were observed. The hypothesis for GSH-adduct formation was driven by the electron-rich nature of the tricyclic core. Herein, we describe our efforts to reduce the electron-rich nature of the core. Ultimately, a strategy focused on core modifications to block metabolic hot spots coupled with substitution pattern changes (C8 N → C linked) led to the identification of new tricyclic analogues with minimal GSH-adduct formation across species while maintaining an overall balanced profile.
© 2022 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
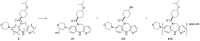

References
-
- Yan H.; Parsons D. W.; Jin G.; McLendon R.; Rasheed B. A.; Yuan W.; Kos I.; Batinic-Haberle I.; Jones S.; Riggins G. J.; Friedman H.; Friedman A.; Reardon D.; Herndon J.; Kinzler K. W.; Velculescu V. E.; Vogelstein B.; Bigner D. D. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009, 360, 765–773. 10.1056/NEJMoa0808710. - DOI - PMC - PubMed
-
- Parsons D. W.; Jones S.; Zhang X.; Lin J. C.; Leary R. J.; Angenendt P.; Mankoo P.; Carter H.; Siu I. M.; Gallia G. L.; Olivi A.; McLendon R.; Rasheed B. A.; Keir S.; Nikolskaya T.; Nikolsky Y.; Busam D. A.; Tekleab H.; Diaz L. A. Jr; Hartigan J.; Smith D. R.; Strausberg R. L.; Marie S. K.; Shinjo S. M.; Yan H.; Riggins G. J.; Bigner D. D.; Karchin R.; Papadopoulos N.; Parmigiani G.; Vogelstein B.; Velculescu V. E.; Kinzler K. W. An integrated genomic analysis of human glioblastoma multiforme. Science 2008, 321, 1807–1812. 10.1126/science.1164382. - DOI - PMC - PubMed
-
- Paschka P.; Schlenk R. F.; Gaidzik V. I.; Habdank M.; Krönke J.; Bullinger L.; Späth D.; Kayser S.; Zucknick M.; Götze K.; Horst H. A.; Germing U.; Döhner H.; Döhner K. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J. Clin. Oncol. 2010, 28, 3636–3643. 10.1200/JCO.2010.28.3762. - DOI - PubMed
-
- Ward P. S.; Patel J.; Wise D. R.; Abdel-Wahab O.; Bennett B. D.; Coller H. A.; Cross J. R.; Fantin V. R.; Hedvat C. V.; Perl A. E.; Rabinowitz J. D.; Carroll M.; Su S. M.; Sharp K. A.; Levine R. L.; Thompson C. B. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alphaketoglutarate to 2-hydroxyglutarate. Cancer Cell 2010, 17, 225–234. 10.1016/j.ccr.2010.01.020. - DOI - PMC - PubMed
-
- Dang L.; White D. W.; Gross S.; Bennett B. D.; Bittinger M. A.; Driggers E. M.; Fantin V. R.; Jang H. G.; Jin S.; Keenan M. C.; Marks K. M.; Prins R. M.; Ward P. S.; Yen K. E.; Liau L. M.; Rabinowitz J. D.; Cantley L. C.; Thompson C. B.; Vander Heiden M. G.; Su S. M. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009, 462, 739–744. 10.1038/nature08617. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

