Evaluation of Low-Dose Aspirin use among Critically Ill Patients with COVID-19: A Multicenter Propensity Score Matched Study
- PMID: 35450493
- PMCID: PMC9038962
- DOI: 10.1177/08850666221093229
Evaluation of Low-Dose Aspirin use among Critically Ill Patients with COVID-19: A Multicenter Propensity Score Matched Study
Abstract
Background: Aspirin is widely used as a cardioprotective agent due to its antiplatelet and anti-inflammatory properties. The literature has assessed and evaluated its role in hospitalized COVID-19 patients. However, no data are available regarding its role in COVID-19 critically ill patients. This study aimed to evaluate the use of low-dose aspirin (81-100 mg) and its impact on outcomes in critically ill patients with COVID-19.
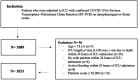
Method: A multicenter, retrospective cohort study of all critically ill adult patients with confirmed COVID-19 admitted to intensive care units (ICUs) between March 1, 2020, and March 31, 2021. Eligible patients were classified into two groups based on aspirin use during ICU stay. The primary outcome was in-hospital mortality, and other outcomes were considered secondary. Propensity score matching was used (1:1 ratio) based on the selected criteria.
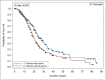
Results: A total of 1033 patients were eligible, and 352 patients were included after propensity score matching. The in-hospital mortality (HR 0.73 [0.56, 0.97], p = 0.03) was lower in patients who received aspirin during stay. Conversely, patients who received aspirin had a higher odds of major bleeding than those in the control group (OR 2.92 [0.91, 9.36], p = 0.07); however, this was not statistically significant. Additionally, subgroup analysis showed a possible mortality benefit for patients who used aspirin therapy prior to hospitalization and continued during ICU stay (HR 0.72 [0.52, 1.01], p = 0.05), but not with the new initiation of aspirin (HR 1.22 [0.68, 2.20], p = 0.50).
Conclusion: Continuation of aspirin therapy during ICU stay in critically ill patients with COVID-19 who were receiving it prior to ICU admission may have a mortality benefit; nevertheless, it may be associated with an increased risk of significant bleeding. Appropriate evaluation for safety versus benefits of utilizing aspirin therapy during ICU stay in COVID19 critically ill patients is highly recommended.
Keywords: 30-day mortality; COVID-19; SARS-coV-2; aspirin; critically ill; in-hospital mortality; intensive care units (ICUs).
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous