Insights into the Cellular Interactions and Molecular Mechanisms of Ketogenic Diet for Comprehensive Management of Epilepsy
- PMID: 35450526
- PMCID: PMC9886834
- DOI: 10.2174/1570159X20666220420130109
Insights into the Cellular Interactions and Molecular Mechanisms of Ketogenic Diet for Comprehensive Management of Epilepsy
Abstract
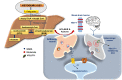
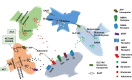
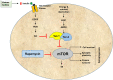
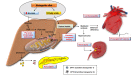
A high-fat diet with appropriate protein and low carbohydrate content, widely known as the ketogenic diet (KD), is considered as an effective non-pharmacotherapeutic treatment option for certain types of epilepsies. Several preclinical and clinical studies have been carried out to elucidate its mechanism of antiepileptic action. Ketone bodies produced after KD's breakdown interact with cellular excito-inhibitory processes and inhibit abnormal neuronal firing. The generated ketone bodies decrease glutamate release by inhibiting the vesicular glutamate transporter 1 and alter the transmembrane potential by hyperpolarization. Apart from their effect on the well-known pathogenic mechanisms of epilepsy, some recent studies have shown the interaction of KD metabolites with novel neuronal targets, particularly adenosine receptors, adenosine triphosphate-sensitive potassium channel, mammalian target of rapamycin, histone deacetylase, hydroxycarboxylic acid receptors, and the NLR family pyrin domain containing 3 inflammasomes to suppress seizures. The role of KD in augmenting gut microbiota as a potential mechanism for epileptic seizure suppression has been established. Furthermore, some recent findings also support the beneficial effect of KD against epilepsy- associated comorbidities. Despite several advantages of the KD in epilepsy management, its use is also associated with a wide range of side effects. Hypoglycemia, excessive ketosis, acidosis, renal stones, cardiomyopathies, and other metabolic disturbances are the primary adverse effects observed with the use of KD. However, in some recent studies, modified KD has been tested with lesser side effects and better tolerability. The present review discusses the molecular mechanism of KD and its role in managing epilepsy and its associated comorbidities.
Keywords: Epilepsy-associated comorbidities; gut microbiota; mammalian target for rapamycin; medium-chain triglyceride; neuronal activity; vesicular glutamate transporters.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Figures
Similar articles
-
Do ketone bodies mediate the anti-seizure effects of the ketogenic diet?Neuropharmacology. 2018 May 1;133:233-241. doi: 10.1016/j.neuropharm.2018.01.011. Epub 2018 Jan 8. Neuropharmacology. 2018. PMID: 29325899 Free PMC article. Review.
-
Control of seizures by ketogenic diet-induced modulation of metabolic pathways.Amino Acids. 2017 Jan;49(1):1-20. doi: 10.1007/s00726-016-2336-7. Epub 2016 Sep 28. Amino Acids. 2017. PMID: 27683025 Review.
-
Does the ketogenic ratio matter when using ketogenic diet therapy in pediatric epilepsy?Epilepsia. 2023 Feb;64(2):284-291. doi: 10.1111/epi.17476. Epub 2022 Dec 15. Epilepsia. 2023. PMID: 36471628 Review.
-
Mechanisms of Ketogenic Diet Action.In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 79. In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 79. PMID: 39637124 Free Books & Documents. Review.
-
The Metabolic Role of Ketogenic Diets in Treating Epilepsy.Nutrients. 2022 Nov 29;14(23):5074. doi: 10.3390/nu14235074. Nutrients. 2022. PMID: 36501104 Free PMC article. Review.
Cited by
-
D-beta-hydroxybutyrate protects against microglial activation in lipopolysaccharide-treated mice and BV-2 cells.Metab Brain Dis. 2023 Mar;38(3):1115-1126. doi: 10.1007/s11011-022-01146-7. Epub 2022 Dec 22. Metab Brain Dis. 2023. PMID: 36543978
-
Cancer Metabolism as a Therapeutic Target and Review of Interventions.Nutrients. 2023 Oct 1;15(19):4245. doi: 10.3390/nu15194245. Nutrients. 2023. PMID: 37836529 Free PMC article. Review.
-
Evaluation of the Effect of Parenting Style and Parental Mealtime Actions on the Eating Behavior of Children with Epilepsy.Nutrients. 2024 May 2;16(9):1384. doi: 10.3390/nu16091384. Nutrients. 2024. PMID: 38732630 Free PMC article.

