Ultramicronized Palmitoylethanolamide and Luteolin Supplement Combined with Olfactory Training to Treat Post-COVID-19 Olfactory Impairment: A Multi-Center Double-Blinded Randomized Placebo- Controlled Clinical Trial
- PMID: 35450527
- PMCID: PMC9886808
- DOI: 10.2174/1570159X20666220420113513
Ultramicronized Palmitoylethanolamide and Luteolin Supplement Combined with Olfactory Training to Treat Post-COVID-19 Olfactory Impairment: A Multi-Center Double-Blinded Randomized Placebo- Controlled Clinical Trial
Abstract
Background: Olfactory training is the only evidence-based treatment for post-viral olfactory dysfunction. Smell disorders after SARS-CoV-2 infection have been attributed to neuroinflammatory events within the olfactory bulb and the central nervous system. Therefore, targeting neuroinflammation is one potential strategy for promoting recovery from post-COVID-19 chronic olfactory dysfunction. Palmitoylethanolamide and luteolin (PEA-LUT) are candidate antiinflammatory/ neuroprotective agents.
Objective: To investigate recovery of olfactory function in patients treated with PEA-LUT oral supplements plus olfactory training versus olfactory training plus placebo.
Methods: Multicenter double-blinded randomized placebo-controlled clinical trial was held. Eligible subjects had prior COVID-19 and persistent olfactory impairment >6 months after follow-up SARS-CoV-2 negative testing, without prior history of olfactory dysfunction or other sinonasal disorders. Participants were randomized to daily oral supplementation with ultramicronized PEA-LUT 770 mg plus olfactory training (intervention group) or olfactory training with placebo (control). Sniffin' Sticks assessments were used to test the patients at baseline and 90 days.
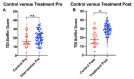
Results: A total of 185 patients, including intervention (130) and control (55) were enrolled. The intervention group showed significantly greater improvement in olfactory threshold, discrimination, and identification scores compared to controls (p=0.0001). Overall, 92% of patients in the intervention group improved versus 42% of controls. Magnitude of recovery was significantly greater in the intervention group versus control (12.8 + 8.2 versus mean 3.2 + 3), with >10-fold higher prevalence of anosmia in control versus intervention groups at the 90-day endpoint.
Conclusion: Among individuals with olfactory dysfunction post-COVID-19, combining PEA-LUT with olfactory training resulted in greater recovery of smell than olfactory training alone.
Keywords: COVID-19; SARS-CoV-2; anosmia; anti-inflammatory; coronavirus; hyposmia; olfaction; olfactory dysfunction; olfactory training.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Figures





References
-
- Gerkin R.C., Ohla K., Veldhuizen M.G., Joseph P.V., Kelly C.E., Bakke A.J., Steele K.E., Farruggia M.C., Pellegrino R., Pepino M.Y., Bouysset C., Soler G.M., Pereda-Loth V., Dibattista M., Cooper K.W., Croijmans I., Di Pizio A., Ozdener M.H., Fjaeldstad A.W., Lin C., Sandell M.A., Singh P.B., Brindha V.E., Olsson S.B., Saraiva L.R., Ahuja G., Alwashahi M.K., Bhutani S., D’errico A., Fornazieri M.A., Golebiowski J., Dar H.L., Öztürk L., Roura E., Spinelli S., Whitcroft K.l., Faraji F., Fischmeister F.P.S., Heinbockel T., Hsieh J.W., Huart C., Konstantinidis I., Menini A., Morini G., Olofsson J.K., Philpott C.M., Pierron D., Shields V.D.C., Voznessenskaya V.V., Albayay J., Altundag A., Bensafi M., Bock M.A., Calcinoni O., Fredborg W., Laudamiel C., Lim J., Lundström J.N., Macchi A., Meyer P., Moein S.T., Santamaría E., Sengupta D., Rohlfs D.P., Yanik H., Hummel T., Hayes J.E., Reed D.R., Niv M.Y., Munger S.D., Parma V. Gccr Group Author. Recent smell loss is the best predictor of Covid-19 Among Individuals With Recent Respiratory Symptoms. Chem. Senses. 2021;46:Bjaa081. - PMC - PubMed
-
- D’Ascanio L., Pandolfini M., Cingolani C., Latini G., Gradoni P., Capalbo M., Frausini G., Maranzano M., Brenner M.J., Di Stadio A. Olfactory Dysfunction in COVID-19 Patients: Prevalence and Prognosis for Recovering Sense of Smell. Otolaryngol. Head Neck Surg. 2021;164(1):82–86. doi: 10.1177/0194599820943530. - DOI - PubMed
-
- Mercante G., Ferreli F., De Virgilio A., Gaino F., Di Bari M., Colombo G., Russo E., Costantino A., Pirola F., Cugini G., Malvezzi L., Morenghi E., Azzolini E., Lagioia M., Spriano G. Prevalence of taste and smell dysfunction in coronavirus disease 2019. JAMA Otolaryngol. Head Neck Surg. 2020;146(8):723–728. doi: 10.1001/jamaoto.2020.1155. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

