New Insights of Early Brain Injury after Subarachnoid Hemorrhage: A Focus on the Caspase Family
- PMID: 35450528
- PMCID: PMC10190145
- DOI: 10.2174/1570159X20666220420115925
New Insights of Early Brain Injury after Subarachnoid Hemorrhage: A Focus on the Caspase Family
Abstract
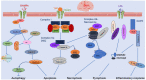
Spontaneous subarachnoid hemorrhage (SAH), primarily caused by ruptured intracranial aneurysms, remains a prominent clinical challenge with a high rate of mortality and morbidity worldwide. Accumulating clinical trials aiming at the prevention of cerebral vasospasm (CVS) have failed to improve the clinical outcome of patients with SAH. Therefore, a growing number of studies have shifted focus to the pathophysiological changes that occur during the periods of early brain injury (EBI). New pharmacological agents aiming to alleviate EBI have become a promising direction to improve outcomes after SAH. Caspases belong to a family of cysteine proteases with diverse functions involved in maintaining metabolism, autophagy, tissue differentiation, regeneration, and neural development. Increasing evidence shows that caspases play a critical role in brain pathology after SAH. Therefore, caspase regulation could be a potential target for SAH treatment. Herein, we provide an overview pertaining to the current knowledge on the role of caspases in EBI after SAH, and we discuss the promising therapeutic value of caspase-related agents after SAH.
Keywords: Subarachnoid hemorrhage; caspase regulation; caspase-related agents; caspases; cerebral vasospasm; early brain injury.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Conflict of interest statement
The authors declare no conflict of interest, financial or otherwise.
Figures

Similar articles
-
Hydrogen-Rich Saline Attenuated Subarachnoid Hemorrhage-Induced Early Brain Injury in Rats by Suppressing Inflammatory Response: Possible Involvement of NF-κB Pathway and NLRP3 Inflammasome.Mol Neurobiol. 2016 Jul;53(5):3462-3476. doi: 10.1007/s12035-015-9242-y. Epub 2015 Jun 20. Mol Neurobiol. 2016. PMID: 26091790
-
Neurovascular events after subarachnoid hemorrhage: focusing on subcellular organelles.Acta Neurochir Suppl. 2015;120:39-46. doi: 10.1007/978-3-319-04981-6_7. Acta Neurochir Suppl. 2015. PMID: 25366597 Free PMC article. Review.
-
The role of autophagy and apoptosis in early brain injury after subarachnoid hemorrhage: an updated review.Mol Biol Rep. 2022 Nov;49(11):10775-10782. doi: 10.1007/s11033-022-07756-9. Epub 2022 Jul 12. Mol Biol Rep. 2022. PMID: 35819555 Review.
-
New Mechanisms and Targets of Subarachnoid Hemorrhage: A Focus on Mitochondria.Curr Neuropharmacol. 2022;20(7):1278-1296. doi: 10.2174/1570159X19666211101103646. Curr Neuropharmacol. 2022. PMID: 34720082 Free PMC article. Review.
-
Biological Effects and Mechanisms of Caspases in Early Brain Injury after Subarachnoid Hemorrhage.Oxid Med Cell Longev. 2022 Jul 5;2022:3345637. doi: 10.1155/2022/3345637. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35847583 Free PMC article. Review.
Cited by
-
Sympathetic nerve block as an add-on therapy for intervention and prevention of cerebral vasospasm after subarachnoid hemorrhage.Front Neurol. 2025 Jun 6;16:1571550. doi: 10.3389/fneur.2025.1571550. eCollection 2025. Front Neurol. 2025. PMID: 40546263 Free PMC article. Review.
-
Mechanisms and interventions in aneurysmal subarachnoid hemorrhage: Unraveling the role of inflammatory responses and cell death in early brain injury (Review).Mol Med Rep. 2025 Sep;32(3):256. doi: 10.3892/mmr.2025.13621. Epub 2025 Jul 19. Mol Med Rep. 2025. PMID: 40682857 Free PMC article. Review.
-
Progress in Research on TLR4-Mediated Inflammatory Response Mechanisms in Brain Injury after Subarachnoid Hemorrhage.Cells. 2022 Nov 26;11(23):3781. doi: 10.3390/cells11233781. Cells. 2022. PMID: 36497041 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

