Age-related brain deviations and aggression
- PMID: 35450543
- PMCID: PMC10325848
- DOI: 10.1017/S003329172200068X
Age-related brain deviations and aggression
Abstract
Background: Disruptive behavior disorders (DBD) are heterogeneous at the clinical and the biological level. Therefore, the aims were to dissect the heterogeneous neurodevelopmental deviations of the affective brain circuitry and provide an integration of these differences across modalities.
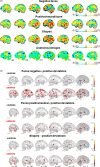
Methods: We combined two novel approaches. First, normative modeling to map deviations from the typical age-related pattern at the level of the individual of (i) activity during emotion matching and (ii) of anatomical images derived from DBD cases (n = 77) and controls (n = 52) aged 8-18 years from the EU-funded Aggressotype and MATRICS consortia. Second, linked independent component analysis to integrate subject-specific deviations from both modalities.
Results: While cases exhibited on average a higher activity than would be expected for their age during face processing in regions such as the amygdala when compared to controls these positive deviations were widespread at the individual level. A multimodal integration of all functional and anatomical deviations explained 23% of the variance in the clinical DBD phenotype. Most notably, the top marker, encompassing the default mode network (DMN) and subcortical regions such as the amygdala and the striatum, was related to aggression across the whole sample.
Conclusions: Overall increased age-related deviations in the amygdala in DBD suggest a maturational delay, which has to be further validated in future studies. Further, the integration of individual deviation patterns from multiple imaging modalities allowed to dissect some of the heterogeneity of DBD and identified the DMN, the striatum and the amygdala as neural signatures that were associated with aggression.
Keywords: Aggression; disruptive behavior disorders; emotion processing; fMRI; normative modeling.
Conflict of interest statement
TB served in an advisory or consultancy role for Actelion, Hexal Pharma, Lilly, Lundbeck, Medice, Novartis and Shire. He received conference support or speaker's fees from Lilly, Medice, Novartis and Shire. He has been involved in clinical trials conducted by Shire and Viforpharma. He received royalties from Hogrefe, Kohlhammer, CIP Medien and Oxford University Press. PS's department has received his speaker's fees from Medice. DB serves as an unpaid scientific consultant for an EU-funded neurofeedback trial unrelated to the present work. AML has received consultant fees from American Association for the Advancement of Science, Atheneum Partners, Blueprint Partnership, Boehringer Ingelheim, Daimler und Benz Stiftung, Elsevier, F. Hoffmann-La Roche, ICARE Schizophrenia, K. G. Jebsen Foundation, L.E.K Consulting, Lundbeck International Foundation (LINF), R. Adamczak, Roche Pharma, Science Foundation, Sumitomo Dainippon Pharma, Synapsis Foundation – Alzheimer Research Switzerland, System Analytics, and has received lectures fees including travel fees from Boehringer Ingelheim, Fama Public Relations, Institut d'investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Janssen-Cilag, Klinikum Christophsbad, Göppingen, Lilly Deutschland, Luzerner Psychiatrie, LVR Klinikum Düsseldorf, LWL Psychiatrie Verbund Westfalen-Lippe, Otsuka Pharmaceuticals, Reunions i Ciencia S. L., Spanish Society of Psychiatry, Südwestrundfunk Fernsehen, Stern TV, and Vitos Klinikum Kurhessen. JKB has been a consultant to, advisory board member of, and a speaker for Takeda/Shire, Medice, Roche, Angelini, and Servier. He is not an employee of any of these companies and not a stock shareholder of any of these companies. He has no other financial or material support, including expert testimony, patents, or royalties. CFB is director and shareholder in SBGneuro Ltd. TC has received consultancy from Roche and received book royalties from Guildford Press and Sage. DM has been a consultant to, and advisory board member, for Roche and Servier. He is not an employee of any of these companies, and not a stock shareholder of any of these companies. CA has been a consultant to or has received honoraria or grants from Acadia, Angelini, Gedeon Richter, Janssen Cilag, Lundbeck, Minerva, Otsuka, Roche, Sage, Servier, Shire, Schering Plough, Sumitomo Dainippon Pharma, Sunovion and Takeda. The present work is unrelated to the above grants and relationships. The other authors report no potential conflicts of interest.
Figures



References
-
- Achenbach, T. M., Howell, C. T., Quay, H. C., & Conners, C. K. (1991). National survey of problems and competencies among four- to sixteen-year-olds: Parents’ reports for normative and clinical samples. Monographs of the Society for Research in Child Development, 56(3), 1–131. Retrieved from https://doi.org/110.1111/j.1540–5834.1991.tb01174.x. - PubMed
-
- Aggensteiner, P. M., Holz, N. E., Bottinger, B. W., Baumeister, S., Hohmann, S., Werhahn, J. E., … Brandeis, D. (2020). The effects of callous-unemotional traits and aggression subtypes on amygdala activity in response to negative faces. Psychological Medicine, 52(3), 476–484. doi:10.1017/S0033291720002111. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

