Study protocol: the TRAnsplant BIOpsies (TRABIO) study - a prospective, observational, multicentre cohort study to assess the treatment of kidney graft rejections
- PMID: 35450886
- PMCID: PMC9024278
- DOI: 10.1136/bmjopen-2020-048122
Study protocol: the TRAnsplant BIOpsies (TRABIO) study - a prospective, observational, multicentre cohort study to assess the treatment of kidney graft rejections
Abstract
Introduction: Despite continued efforts, long-term outcomes of kidney transplantation remain unsatisfactory. Kidney graft rejections are independent risk factors for graft failure. At the participating centres of the TRAnsplant BIOpsies study group, a common therapeutic standard has previously been defined for the treatment of graft rejections. The outcomes of this strategy will be assessed in a prospective, observational cohort study.
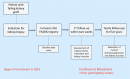
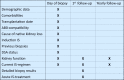
Methods and analysis: A total of 800 kidney transplantation patients will be enrolled who undergo a graft biopsy because of deteriorating kidney function. Patients will be stratified according to the Banff classification, and the influence of the treatment strategy on end points will be assessed using regression analysis. Primary end points will be all-cause mortality and graft survival. Secondary end points will be worsening of kidney function (≥30% decline of estimated Glomerular Filtration Rate [eGFR] or new-onset large proteinuria), recurrence of graft rejection and treatment response. Baseline data and detailed histopathology data will be entered into an electronic database on enrolment. During a first follow-up period (within 14 days) and subsequent yearly follow-ups (for 5 years), treatment strategies and clinical course will be recorded. Recruitment at the four participating centres started in September 2016. As of August 2020, 495 patients have been included.
Ethics and dissemination: Ethical approval for the study has been obtained from the ethics committee of Kiel (AZ B 278/16) and was confirmed by the committees of Munich, Mainz and Stuttgart. The results will be reported in a peer-reviewed journal, according to the Strengthening the Reporting of Observational Studies in Epidemiology criteria.
Trial registration number: ISRCTN78772632; Pre-results.
Keywords: Nephrology; Renal transplantation; TRANSPLANT MEDICINE; Transplant pathology.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JW-M has received research support from GSK, DIAMED, Miltenyi Biotech as well as personal fees from Novartis, GSK, Boehringer-Ingelheim, Miltenyi, Vifor and Chiesi (all unrelated to the submitted work).
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
