Epidemiology of adverse drug events and medication errors in four nursing homes in Japan: the Japan Adverse Drug Events (JADE) Study
- PMID: 35450935
- PMCID: PMC9685701
- DOI: 10.1136/bmjqs-2021-014280
Epidemiology of adverse drug events and medication errors in four nursing homes in Japan: the Japan Adverse Drug Events (JADE) Study
Abstract
Background: Worldwide, the emergence of super-ageing societies has increased the number of older people requiring support for daily activities. Many elderly residents of nursing homes (NHs) take drugs to treat chronic conditions; however, there are few reports of medication safety in NHs, especially from non-western countries.
Objective: We examined the incidence and nature of adverse drug events (ADEs) and medication errors (MEs) in NHs for the elderly in Japan.
Design, setting, and participants: The Japan Adverse Drug Events Study for NHs is a prospective cohort study that was conducted among all residents, except for short-term admissions, at four NHs for older people in Japan for 1 year.
Measurements: Trained physicians and psychologists, five and six in number, respectively, reviewed all charts of the residents to identify suspected ADEs and MEs, which were then classified by the physicians into ADEs, potential ADEs and other MEs after the exclusion of ineligible events, for the assessment of their severity and preventability. The kappa score for presence of an ADE and preventability were 0.89 and 0.79, respectively.
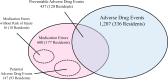
Results: We enrolled 459 residents, and this yielded 3315 resident-months of observation time. We identified 1207 ADEs and 600 MEs (incidence: 36.4 and 18.1 per 100 resident-months, respectively) during the study period. About one-third of ADEs were preventable, and MEs were most frequently observed in the monitoring stage (72%, 433/600), with 71% of the MEs occurring due to inadequate observation following the physician's prescription.
Conclusion: In Japan, ADEs and MEs are common among elderly residents of NHs. The assessment and appropriate adjustment of medication preadmission and postadmission to NHs are needed to improve medication safety, especially when a single physician is responsible for prescribing most medications for the residents, as is usually the case in Japan.
Keywords: Adverse events, epidemiology and detection; Medical error, measurement/epidemiology; Medication safety; Nursing homes; Pharmacoepidemiology.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Comment in
-
Medication safety in nursing home patients.BMJ Qual Saf. 2022 Dec;31(12):849-852. doi: 10.1136/bmjqs-2022-014791. Epub 2022 Jul 5. BMJ Qual Saf. 2022. PMID: 35790384 No abstract available.
References
-
- United Nations, Department of Economic and Social Affairs, Population Division . World population prospects 2019, online edition. Rev. 1, 2019. Available: https://population.un.org/wpp/Download/Standard/Population/ [Accessed May 2019].
-
- Japanese Ministry of Health Labour and Welfare . Long-term care insurance annual report in 2018. Available: https://www.mhlw.go.jp/topics/kaigo/osirase/jigyo/18/index.html [Accessed 21 May 2021].
-
- Japanese Ministry of Health Labour and Welfare . Social security Council. Long-term care insurance Subcommittee (81st meeting), reference material 2018: 8. Available: https://www.mhlw.go.jp/content/12300000/000547178.pdf [Accessed 21 May 2021].
-
- Centers for Disease Control and Prevention . Long-term care providers and services users in the United States: data from the National study of long-term care providers, 2015–2016. Available: https://www.cdc.gov/nchs/data/series/sr_03/sr03_43-508.pdf [Accessed 21 May 2021].
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous