Use of Real-World Data and Physiologically-Based Pharmacokinetic Modeling to Characterize Enoxaparin Disposition in Children With Obesity
- PMID: 35451072
- PMCID: PMC9504927
- DOI: 10.1002/cpt.2618
Use of Real-World Data and Physiologically-Based Pharmacokinetic Modeling to Characterize Enoxaparin Disposition in Children With Obesity
Abstract
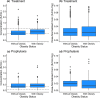
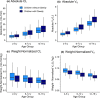
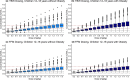
Dosing guidance for children with obesity is often unknown despite the fact that nearly 20% of US children are classified as obese. Enoxaparin, a commonly prescribed low-molecular-weight heparin, is dosed based on body weight irrespective of obesity status to achieve maximum concentration within a narrow therapeutic or prophylactic target range. However, whether children with and without obesity experience equivalent enoxaparin exposure remains unclear. To address this clinical question, 2,825 anti-activated factor X (anti-Xa) surrogate concentrations were collected from the electronic health records of 596 children, including those with obesity. Using linear mixed-effects regression models, we observed that 4-hour anti-Xa concentrations were statistically significantly different in children with and without obesity, even for children with the same absolute dose (P = 0.004). To further mechanistically explore obesity-associated differences in anti-Xa concentration, a pediatric physiologically-based pharmacokinetic (PBPK) model was developed in adults, and then scaled to children with and without obesity. This PBPK model incorporated binding of enoxaparin to antithrombin to form anti-Xa and elimination via heparinase-mediated metabolism and glomerular filtration. Following scaling, the PBPK model predicted real-world pediatric concentrations well, with an average fold error (standard deviation of the fold error) of 0.82 (0.23) and 0.87 (0.26) in children with and without obesity, respectively. PBPK model simulations revealed that children with obesity have at most 20% higher 4-hour anti-Xa concentrations under recommended, total body weight-based dosing compared to children without obesity owing to reduced weight-normalized clearance. Enoxaparin exposure was better matched across age groups and obesity status using fat-free mass weight-based dosing.
© 2022 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
D.G. receives research support from Nabriva Therapeutics through a contract with the University of North Carolina at Chapel Hill. In addition, D.G. serves as a consultant for Tellus Therapeutics, focusing on neonatal drug development. All other coauthors declared no competing interests for this work.
Figures
References
-
- Lovenox [product monograph] <https://pdf.hres.ca/dpd_pm/00047708.PDF> (2018). Accessed May 27, 2020.
-
- Geerts, W.H. et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence‐based clinical practice guidelines (8th edition). Chest 133, 381S–453S (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

