Influenza antivirals and animal models
- PMID: 35451200
- PMCID: PMC9157400
- DOI: 10.1002/2211-5463.13416
Influenza antivirals and animal models
Abstract
Influenza A and B viruses are among the most prominent human respiratory pathogens. About 3-5 million severe cases of influenza are associated with 300 000-650 000 deaths per year globally. Antivirals effective at reducing morbidity and mortality are part of the first line of defense against influenza. FDA-approved antiviral drugs currently include adamantanes (rimantadine and amantadine), neuraminidase inhibitors (NAI; peramivir, zanamivir, and oseltamivir), and the PA endonuclease inhibitor (baloxavir). Mutations associated with antiviral resistance are common and highlight the need for further improvement and development of novel anti-influenza drugs. A summary is provided for the current knowledge of the approved influenza antivirals and antivirals strategies under evaluation in clinical trials. Preclinical evaluations of novel compounds effective against influenza in different animal models are also discussed.
Keywords: animal models; antiviral-host factors; antivirals; influenza; mice; neuraminidase inhibitors.
© 2022 The Authors. FEBS Open Bio published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
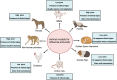
Figures

References
-
- O'Hanlon R, Shaw ML. Baloxavir marboxil: the new influenza drug on the market. Curr Opin Virol. 2019;35:14–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

