SARS-CoV-2 productively infects primary human immune system cells in vitro and in COVID-19 patients
- PMID: 35451490
- PMCID: PMC9384834
- DOI: 10.1093/jmcb/mjac021
SARS-CoV-2 productively infects primary human immune system cells in vitro and in COVID-19 patients
Abstract
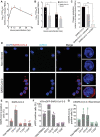
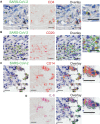
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is associated with a hyperinflammatory state and lymphocytopenia, a hallmark that appears as both signature and prognosis of disease severity outcome. Although cytokine storm and a sustained inflammatory state are commonly associated with immune cell depletion, it is still unclear whether direct SARS-CoV-2 infection of immune cells could also play a role in this scenario by harboring viral replication. We found that monocytes, as well as both B and T lymphocytes, were susceptible to SARS-CoV-2 infection in vitro, accumulating double-stranded RNA consistent with viral RNA replication and ultimately leading to expressive T cell apoptosis. In addition, flow cytometry and immunofluorescence analysis revealed that SARS-CoV-2 was frequently detected in monocytes and B lymphocytes from coronavirus disease 2019 (COVID-19) patients. The rates of SARS-CoV-2-infected monocytes in peripheral blood mononuclear cells from COVID-19 patients increased over time from symptom onset, with SARS-CoV-2-positive monocytes, B cells, and CD4+ T lymphocytes also detected in postmortem lung tissue. These results indicated that SARS-CoV-2 infection of blood-circulating leukocytes in COVID-19 patients might have important implications for disease pathogenesis and progression, immune dysfunction, and virus spread within the host.
Keywords: COVID-19; SARS-CoV-2; apoptosis; lymphocytes; lymphocytopenia; monocytes; peripheral blood mononuclear cell (PBMC).
© The Author(s) (2022). Published by Oxford University Press on behalf of Journal of Molecular Cell Biology, CEMCS, CAS.
Figures



Update of
-
Infection of human lymphomononuclear cells by SARS-CoV-2.bioRxiv [Preprint]. 2020 Aug 7:2020.07.28.225912. doi: 10.1101/2020.07.28.225912. bioRxiv. 2020. Update in: J Mol Cell Biol. 2022 Aug 17;14(4):mjac021. doi: 10.1093/jmcb/mjac021. PMID: 34013264 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

