High-efficiency nonviral CRISPR/Cas9-mediated gene editing of human T cells using plasmid donor DNA
- PMID: 35452075
- PMCID: PMC9040063
- DOI: 10.1084/jem.20211530
High-efficiency nonviral CRISPR/Cas9-mediated gene editing of human T cells using plasmid donor DNA
Abstract
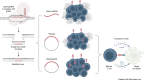
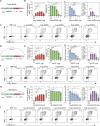
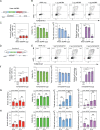
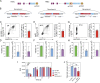
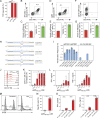
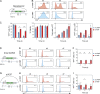
Genome engineering of T lymphocytes, the main effectors of antitumor adaptive immune responses, has the potential to uncover unique insights into their functions and enable the development of next-generation adoptive T cell therapies. Viral gene delivery into T cells, which is currently used to generate CAR T cells, has limitations in regard to targeting precision, cargo flexibility, and reagent production. Nonviral methods for effective CRISPR/Cas9-mediated gene knock-out in primary human T cells have been developed, but complementary techniques for nonviral gene knock-in can be cumbersome and inefficient. Here, we report a convenient and scalable nonviral method that allows precise gene edits and transgene integration in primary human T cells, using plasmid donor DNA template and Cas9-RNP. This method is highly efficient for single and multiplex gene manipulation, without compromising T cell function, and is thus valuable for use in basic and translational research.
© 2022 Genentech, Inc.
Conflict of interest statement
Disclosures: S.A. Oh, K. Senger, S. Madireddi, D. Shaw, B. Haley, and S. Rutz reported a provisional patent pending. All authors are fulltime employees of Genentech, Inc. and shareholders of Roche. No other disclosures were reported.
Figures







References
-
- Bloemberg, D., Nguyen T., MacLean S., Zafer A., Gadoury C., Gurnani K., Chattopadhyay A., Ash J., Lippens J., Harcus D., et al. . 2020. A high-throughput method for characterizing novel chimeric antigen receptors in jurkat cells. Mol. Ther. Methods Clin. Dev. 16:238–254. 10.1016/j.omtm.2020.01.012 - DOI - PMC - PubMed
-
- Choi, B.D., Yu X., Castano A.P., Darr H., Henderson D.B., Bouffard A.A., Larson R.C., Scarfò I., Bailey S.R., Gerhard G.M., et al. . 2019. CRISPR-Cas9 disruption of PD-1 enhances activity of universal EGFRvIII CAR T cells in a preclinical model of human glioblastoma. J. Immunother. Cancer. 7:304. 10.1186/s40425-019-0806-7 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

